Piperacillin-Tazobactam Compared With Cefoxitin as Antimicrobial Prophylaxis for Pancreatoduodenectomy: A Randomized Clinical Trial
- PMID: 37078771
- PMCID: PMC10119777
- DOI: 10.1001/jama.2023.5728
Piperacillin-Tazobactam Compared With Cefoxitin as Antimicrobial Prophylaxis for Pancreatoduodenectomy: A Randomized Clinical Trial
Abstract
Importance: Despite improvements in perioperative mortality, the incidence of postoperative surgical site infection (SSI) remains high after pancreatoduodenectomy. The effect of broad-spectrum antimicrobial surgical prophylaxis in reducing SSI is poorly understood.
Objective: To define the effect of broad-spectrum perioperative antimicrobial prophylaxis on postoperative SSI incidence compared with standard care antibiotics.
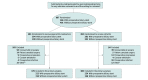
Design, setting, and participants: Pragmatic, open-label, multicenter, randomized phase 3 clinical trial at 26 hospitals across the US and Canada. Participants were enrolled between November 2017 and August 2021, with follow-up through December 2021. Adults undergoing open pancreatoduodenectomy for any indication were eligible. Individuals were excluded if they had allergies to study medications, active infections, chronic steroid use, significant kidney dysfunction, or were pregnant or breastfeeding. Participants were block randomized in a 1:1 ratio and stratified by the presence of a preoperative biliary stent. Participants, investigators, and statisticians analyzing trial data were unblinded to treatment assignment.
Intervention: The intervention group received piperacillin-tazobactam (3.375 or 4 g intravenously) as perioperative antimicrobial prophylaxis, while the control group received cefoxitin (2 g intravenously; standard care).
Main outcomes and measures: The primary outcome was development of postoperative SSI within 30 days. Secondary end points included 30-day mortality, development of clinically relevant postoperative pancreatic fistula, and sepsis. All data were collected as part of the American College of Surgeons National Surgical Quality Improvement Program.
Results: The trial was terminated at an interim analysis on the basis of a predefined stopping rule. Of 778 participants (378 in the piperacillin-tazobactam group [median age, 66.8 y; 233 {61.6%} men] and 400 in the cefoxitin group [median age, 68.0 y; 223 {55.8%} men]), the percentage with SSI at 30 days was lower in the perioperative piperacillin-tazobactam vs cefoxitin group (19.8% vs 32.8%; absolute difference, -13.0% [95% CI, -19.1% to -6.9%]; P < .001). Participants treated with piperacillin-tazobactam, vs cefoxitin, had lower rates of postoperative sepsis (4.2% vs 7.5%; difference, -3.3% [95% CI, -6.6% to 0.0%]; P = .02) and clinically relevant postoperative pancreatic fistula (12.7% vs 19.0%; difference, -6.3% [95% CI, -11.4% to -1.2%]; P = .03). Mortality rates at 30 days were 1.3% (5/378) among participants treated with piperacillin-tazobactam and 2.5% (10/400) among those receiving cefoxitin (difference, -1.2% [95% CI, -3.1% to 0.7%]; P = .32).
Conclusions and relevance: In participants undergoing open pancreatoduodenectomy, use of piperacillin-tazobactam as perioperative prophylaxis reduced postoperative SSI, pancreatic fistula, and multiple downstream sequelae of SSI. The findings support the use of piperacillin-tazobactam as standard care for open pancreatoduodenectomy.
Trial registration: ClinicalTrials.gov Identifier: NCT03269994.
Conflict of interest statement
Figures

Comment in
-
Informing a Rational Approach to Antimicrobial Prophylaxis in Open Pancreatoduodenectomy.JAMA. 2023 May 9;329(18):1556-1557. doi: 10.1001/jama.2023.6275. JAMA. 2023. PMID: 37078778 No abstract available.
-
Piperacillin-Tazobactam vs Cefoxitin Prophylaxis for Pancreatoduodenectomy.JAMA. 2023 Sep 19;330(11):1098. doi: 10.1001/jama.2023.12773. JAMA. 2023. PMID: 37721616 No abstract available.
-
Piperacillin-Tazobactam vs Cefoxitin Prophylaxis for Pancreatoduodenectomy.JAMA. 2023 Sep 19;330(11):1098-1099. doi: 10.1001/jama.2023.12770. JAMA. 2023. PMID: 37721617 No abstract available.
Comment on
-
Informing a Rational Approach to Antimicrobial Prophylaxis in Open Pancreatoduodenectomy.JAMA. 2023 May 9;329(18):1556-1557. doi: 10.1001/jama.2023.6275. JAMA. 2023. PMID: 37078778 No abstract available.
References
-
- Beane JD, Borrebach JD, Zureikat AH, Kilbane EM, Thompson VM, Pitt HA. Optimal pancreatic surgery: are we making progress in North America? Ann Surg. 2021;274(4):e355-e363. - PubMed

