Candida albicans Hyphal Morphogenesis within Macrophages Does Not Require Carbon Dioxide or pH-Sensing Pathways
- PMID: 37078861
- PMCID: PMC10187119
- DOI: 10.1128/iai.00087-23
Candida albicans Hyphal Morphogenesis within Macrophages Does Not Require Carbon Dioxide or pH-Sensing Pathways
Abstract
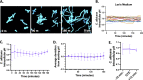
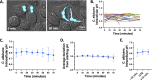
The opportunistic fungal pathogen Candida albicans has evolved a variety of mechanisms for surviving inside and escaping macrophages, including the initiation of filamentous growth. Although several distinct models have been proposed to explain this process at the molecular level, the signals driving hyphal morphogenesis in this context have yet to be clarified. Here, we evaluate the following three molecular signals as potential hyphal inducers within macrophage phagosomes: CO2, intracellular pH, and extracellular pH. Additionally, we revisit previous work suggesting that the intracellular pH of C. albicans fluctuates in tandem with morphological changes in vitro. Using time-lapse microscopy, we observed that C. albicans mutants lacking components of the CO2-sensing pathway were able to undergo hyphal morphogenesis within macrophages. Similarly, a rim101Δ strain was competent in hyphal induction, suggesting that neutral/alkaline pH sensing is not necessary for the initiation of morphogenesis within phagosomes either. Contrary to previous findings, single-cell pH-tracking experiments revealed that the cytosolic pH of C. albicans remains tightly regulated both within macrophage phagosomes and under a variety of in vitro conditions throughout the process of morphogenesis. This finding suggests that intracellular pH is not a signal contributing to morphological changes.
Keywords: Candida; host-pathogen interactions; macrophages; morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
The Mystery of Candida albicans Hyphal Morphogenesis in the Macrophage Phagolysosome.Infect Immun. 2023 May 16;91(5):e0010423. doi: 10.1128/iai.00104-23. Epub 2023 Apr 27. Infect Immun. 2023. PMID: 37129514 Free PMC article.
Similar articles
-
Modulation of phagosomal pH by Candida albicans promotes hyphal morphogenesis and requires Stp2p, a regulator of amino acid transport.PLoS Pathog. 2014 Mar 13;10(3):e1003995. doi: 10.1371/journal.ppat.1003995. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24626429 Free PMC article.
-
The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH.mBio. 2011 May 17;2(3):e00055-11. doi: 10.1128/mBio.00055-11. Print 2011. mBio. 2011. PMID: 21586647 Free PMC article.
-
Glutamate dehydrogenase (Gdh2)-dependent alkalization is dispensable for escape from macrophages and virulence of Candida albicans.PLoS Pathog. 2020 Sep 16;16(9):e1008328. doi: 10.1371/journal.ppat.1008328. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32936835 Free PMC article.
-
Transcriptional control of hyphal morphogenesis in Candida albicans.FEMS Yeast Res. 2020 Feb 1;20(1):foaa005. doi: 10.1093/femsyr/foaa005. FEMS Yeast Res. 2020. PMID: 31981355 Free PMC article. Review.
-
Hyphal development in Candida albicans from different cell states.Curr Genet. 2018 Dec;64(6):1239-1243. doi: 10.1007/s00294-018-0845-5. Epub 2018 May 23. Curr Genet. 2018. PMID: 29796903 Review.
Cited by
-
The Mystery of Candida albicans Hyphal Morphogenesis in the Macrophage Phagolysosome.Infect Immun. 2023 May 16;91(5):e0010423. doi: 10.1128/iai.00104-23. Epub 2023 Apr 27. Infect Immun. 2023. PMID: 37129514 Free PMC article.
-
Candida auris-macrophage cellular interactions and transcriptional response.Infect Immun. 2023 Nov 16;91(11):e0027423. doi: 10.1128/iai.00274-23. Epub 2023 Oct 10. Infect Immun. 2023. PMID: 37815367 Free PMC article.
-
Evolution and strain diversity advance exploration of Candida albicans biology.mSphere. 2024 Aug 28;9(8):e0064123. doi: 10.1128/msphere.00641-23. Epub 2024 Jul 16. mSphere. 2024. PMID: 39012122 Free PMC article. Review.
-
Host-derived reactive oxygen species trigger activation of the Candida albicans transcription regulator Rtg1/3.PLoS Pathog. 2023 Sep 28;19(9):e1011692. doi: 10.1371/journal.ppat.1011692. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37769015 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

