Allosteric modulation of GluN1/GluN3 NMDA receptors by GluN1-selective competitive antagonists
- PMID: 37078900
- PMCID: PMC10125900
- DOI: 10.1085/jgp.202313340
Allosteric modulation of GluN1/GluN3 NMDA receptors by GluN1-selective competitive antagonists
Abstract
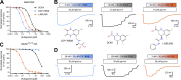
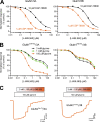
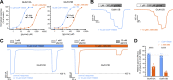
NMDA-type ionotropic glutamate receptors are critical for normal brain function and are implicated in central nervous system disorders. Structure and function of NMDA receptors composed of GluN1 and GluN3 subunits are less understood compared to those composed of GluN1 and GluN2 subunits. GluN1/3 receptors display unusual activation properties in which binding of glycine to GluN1 elicits strong desensitization, while glycine binding to GluN3 alone is sufficient for activation. Here, we explore mechanisms by which GluN1-selective competitive antagonists, CGP-78608 and L-689,560, potentiate GluN1/3A and GluN1/3B receptors by preventing glycine binding to GluN1. We show that both CGP-78608 and L-689,560 prevent desensitization of GluN1/3 receptors, but CGP-78608-bound receptors display higher glycine potency and efficacy at GluN3 subunits compared to L-689,560-bound receptors. Furthermore, we demonstrate that L-689,560 is a potent antagonist of GluN1FA+TL/3A receptors, which are mutated to abolish glycine binding to GluN1, and that this inhibition is mediated by a non-competitive mechanism involving binding to the mutated GluN1 agonist binding domain (ABD) to negatively modulate glycine potency at GluN3A. Molecular dynamics simulations reveal that CGP-78608 and L-689,560 binding or mutations in the GluN1 glycine binding site promote distinct conformations of the GluN1 ABD, suggesting that the GluN1 ABD conformation influences agonist potency and efficacy at GluN3 subunits. These results uncover the mechanism that enables activation of native GluN1/3A receptors by application of glycine in the presence of CGP-78608, but not L-689,560, and demonstrate strong intra-subunit allosteric interactions in GluN1/3 receptors that may be relevant to neuronal signaling in brain function and disease.
© 2023 Rouzbeh et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures









Comment in
-
Activation of excitatory glycine NMDA receptors: At the mercy of a whimsical GluN1 subunit.J Gen Physiol. 2023 Jun 5;155(6):e202313391. doi: 10.1085/jgp.202313391. Epub 2023 May 3. J Gen Physiol. 2023. PMID: 37133818 Free PMC article.
Similar articles
-
Negative allosteric modulation of GluN1/GluN3 NMDA receptors.Neuropharmacology. 2020 Oct 1;176:108117. doi: 10.1016/j.neuropharm.2020.108117. Epub 2020 May 7. Neuropharmacology. 2020. PMID: 32389749 Free PMC article.
-
Structure-based discovery of antagonists for GluN3-containing N-methyl-D-aspartate receptors.Neuropharmacology. 2013 Dec;75:324-36. doi: 10.1016/j.neuropharm.2013.08.003. Epub 2013 Aug 22. Neuropharmacology. 2013. PMID: 23973313 Free PMC article.
-
The N-terminal domain of the GluN3A subunit determines the efficacy of glycine-activated NMDA receptors.Neuropharmacology. 2016 Jun;105:133-141. doi: 10.1016/j.neuropharm.2016.01.014. Epub 2016 Jan 9. Neuropharmacology. 2016. PMID: 26777280
-
Glycine agonism in ionotropic glutamate receptors.Neuropharmacology. 2021 Aug 1;193:108631. doi: 10.1016/j.neuropharm.2021.108631. Epub 2021 May 28. Neuropharmacology. 2021. PMID: 34058193 Review.
-
The GluN3-containing NMDA receptors.Channels (Austin). 2025 Dec;19(1):2490308. doi: 10.1080/19336950.2025.2490308. Epub 2025 Apr 16. Channels (Austin). 2025. PMID: 40235311 Free PMC article. Review.
Cited by
-
Structural prediction of GluN3 NMDA receptors.Front Physiol. 2024 Aug 20;15:1446459. doi: 10.3389/fphys.2024.1446459. eCollection 2024. Front Physiol. 2024. PMID: 39229618 Free PMC article.
-
Atypical antipsychotic drug olanzapine inhibits 5-HT3 receptor-mediated currents by allosteric and non-competitive mechanisms.Korean J Physiol Pharmacol. 2025 Jul 1;29(4):431-442. doi: 10.4196/kjpp.24.340. Epub 2024 Dec 18. Korean J Physiol Pharmacol. 2025. PMID: 39690469 Free PMC article.
-
Discovery of selective GluN1/GluN3A NMDA receptor inhibitors using integrated AI and physics-based approaches.Acta Pharmacol Sin. 2025 Jul 14. doi: 10.1038/s41401-025-01607-6. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40659855
-
Activation of excitatory glycine NMDA receptors: At the mercy of a whimsical GluN1 subunit.J Gen Physiol. 2023 Jun 5;155(6):e202313391. doi: 10.1085/jgp.202313391. Epub 2023 May 3. J Gen Physiol. 2023. PMID: 37133818 Free PMC article.
-
Structure and function of GluN1-3A NMDA receptor excitatory glycine receptor channel.Sci Adv. 2024 Apr 12;10(15):eadl5952. doi: 10.1126/sciadv.adl5952. Epub 2024 Apr 10. Sci Adv. 2024. PMID: 38598639 Free PMC article.
References
-
- Bossi, S., Dhanasobhon D., Ellis-Davies G.C.R., Frontera J., de Brito Van Velze M., Lourenço J., Murillo A., Luján R., Casado M., Perez-Otaño I., et al. . 2022. GluN3A excitatory glycine receptors control adult cortical and amygdalar circuits. Neuron. 110:2438–2454.e8. 10.1016/j.neuron.2022.05.016 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

