Qualitative concept elicitation and cognitive debriefing interviews of symptoms, impacts and selected customized PROMIS® Short Forms: a study in patients with axial spondyloarthritis
- PMID: 37079188
- PMCID: PMC10117270
- DOI: 10.1186/s41687-023-00575-x
Qualitative concept elicitation and cognitive debriefing interviews of symptoms, impacts and selected customized PROMIS® Short Forms: a study in patients with axial spondyloarthritis
Abstract
Background: Sleep disturbance, pain, and fatigue are key symptoms/impacts of axial spondyloarthritis (axSpA). Three customized Patient-Reported Outcomes Measurement Information System (PROMIS®) Short Forms (Sleep Disturbance, Pain Interference, and Fatigue) have been proposed for use in axSpA to assess these key disease concepts. This study was designed to further understand the patient experience of axSpA and evaluate the content validity of the three customized PROMIS® Short Forms to support their use in axSpA clinical trials.
Methods: Non-interventional, cross-sectional, qualitative (concept elicitation [CE] and cognitive debriefing [CD]) study. Participants took part in 90-min telephone interviews. The CE section used open-ended questions to elicit information about axSpA symptoms and impacts. The CD section involved a 'think-aloud' exercise where participants read out each instruction, item, and response option for the customized PROMIS® Short Forms and shared their feedback. Participants also discussed the relevance of the items, response options and recall period. Verbatim interview transcripts were subject to thematic and content analysis.
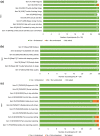
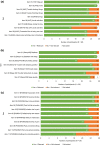
Results: In total, there were 28 participants (non-radiographic axSpA, n = 12; ankylosing spondylitis, n = 16), from the US (n = 20) and Germany (n = 8). Mean age was 52.8 years, and 57% were male; mean time since diagnosis was 9.5 years. The CE section identified 12 distinct symptoms that characterized axSpA: pain, sleep problems, fatigue/tiredness, stiffness, swelling, vision/eye issues, restricted body movements, headache/migraine, spasms, change in posture/stature, balance/coordination problems, and numbness. Pain, sleep problems, and fatigue/tiredness were experienced by ≥ 90% of participants, occurring simultaneously and exacerbating one another. Participants reported axSpA impacted their lives across six domains of health-related quality of life (HRQoL): physical functioning (100%), emotional wellbeing (89%), work/volunteering (79%), social functioning (75%), activities of daily living (61%) and cognitive functioning (54%). Impacts were most frequently associated with pain, stiffness, and fatigue. CD showed the PROMIS® instruments were conceptually comprehensive and well understood, with all items relevant to ≥ 50% of participants.
Conclusions: Pain, sleep problems and fatigue are pivotal symptoms of axSpA and associated with HRQoL impacts. These results were used to update a conceptual model of axSpA which was originally developed based on a targeted literature review. Interpretability and content validity of the customized PROMIS® Short Forms were confirmed, with each deemed to adequately assess key impacts associated with axSpA, making them suitable for use in axSpA clinical trials.
Keywords: Cognitive debriefing; Concept elicitation; Qualitative; axSpA.
© 2023. The Author(s).
Conflict of interest statement
AF, JM, CH, MC, and ST are employees of Adelphi Values, which received funding from GSK for the conduct of this study, although not for manuscript development. WN was an employee of Adelphi Values at the time of study conduct. WHC, MB and DP are employees of and/or hold stocks/shares in GSK.
Figures




Similar articles
-
Qualitative and psychometric approaches to evaluate the PROMIS pain interference and sleep disturbance item banks for use in patients with rheumatoid arthritis.J Patient Rep Outcomes. 2021 Jul 6;5(1):52. doi: 10.1186/s41687-021-00318-w. J Patient Rep Outcomes. 2021. PMID: 34228217 Free PMC article.
-
A concept elicitation study to understand the relationship between sleep and pain in rheumatoid arthritis and axial spondyloarthritis.Qual Life Res. 2024 Feb;33(2):373-385. doi: 10.1007/s11136-023-03524-9. Epub 2023 Oct 27. Qual Life Res. 2024. PMID: 37889386 Free PMC article.
-
Content validity of the ASQoL for use in a non-radiographic axial spondyloarthritis population: a qualitative study.Qual Life Res. 2020 Nov;29(11):3155-3166. doi: 10.1007/s11136-020-02552-z. Epub 2020 Jul 1. Qual Life Res. 2020. PMID: 32607793 Free PMC article.
-
Are patient-reported outcome instruments for ankylosing spondylitis fit for purpose for the axial spondyloarthritis patient? A qualitative and psychometric analysis.Rheumatology (Oxford). 2015 Oct;54(10):1842-51. doi: 10.1093/rheumatology/kev125. Epub 2015 May 21. Rheumatology (Oxford). 2015. PMID: 26001635 Review.
-
Development of a Patient-Reported Outcome Measure for Non-Alcoholic Steatohepatitis (NASH-CHECK): Results of a Qualitative Study.Patient. 2021 Sep;14(5):533-543. doi: 10.1007/s40271-020-00485-w. Epub 2020 Dec 18. Patient. 2021. PMID: 33336323 Free PMC article. Review.
Cited by
-
A conceptual model for advanced/metastatic gastric or gastroesophageal junction cancer: a review of qualitative studies and results from patient interviews.BMC Cancer. 2025 Jan 15;25(1):88. doi: 10.1186/s12885-025-13474-9. BMC Cancer. 2025. PMID: 39815188 Free PMC article. Review.
-
The Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) scale in patients with axial spondyloarthritis: psychometric properties and clinically meaningful thresholds for interpretation.J Patient Rep Outcomes. 2024 Aug 12;8(1):92. doi: 10.1186/s41687-024-00769-x. J Patient Rep Outcomes. 2024. PMID: 39133438 Free PMC article. Clinical Trial.
-
Content development for a new item-bank for measuring multifocal contact lens performance.J Patient Rep Outcomes. 2024 Feb 8;8(1):16. doi: 10.1186/s41687-024-00689-w. J Patient Rep Outcomes. 2024. PMID: 38329635 Free PMC article.
-
Causes of Sleep Disturbance in Early ASAS Spondyloarthritis: A Retrospective Long-Term Experience.J Pers Med. 2025 Jan 17;15(1):31. doi: 10.3390/jpm15010031. J Pers Med. 2025. PMID: 39852224 Free PMC article.
References
-
- Rudwaleit M, van der Heijde D, Landewe R, Listing J, Akkoc N, Brandt J, Braun J, Chou CT, Collantes-Estevez E, Dougados M, Huang F, Gu J, Khan MA, Kirazli Y, Maksymowych WP, Mielants H, Sorensen IJ, Ozgocmen S, Roussou E, Valle-Onate R, Weber U, Wei J, Sieper J. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68:777–783. doi: 10.1136/ard.2009.108233. - DOI - PubMed
-
- Sieper J, Holbrook T, Black CM, Wood R, Hu X, Kachroo S. Burden of illness associated with non-radiographic axial spondyloarthritis: a multiperspective European cross-sectional observational study. Clin Exp Rheumatol. 2016;34:975–983. - PubMed
-
- Lopez-Medina C, Ramiro S, van der Heijde D, Sieper J, Dougados M, Molto A. Characteristics and burden of disease in patients with radiographic and non-radiographic axial Spondyloarthritis: a comparison by systematic literature review and meta-analysis. RMD Open. 2019;5:e001108. doi: 10.1136/rmdopen-2019-001108. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

