Seroprevalence of SARS-CoV-2 specific Immunoglobin G antibodies in rural population of Western Maharashtra, India
- PMID: 37079274
- PMCID: PMC10117619
- DOI: 10.7189/jogh.13.06011
Seroprevalence of SARS-CoV-2 specific Immunoglobin G antibodies in rural population of Western Maharashtra, India
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, responsible for the coronavirus disease 2019 (COVID-19) pandemic, has been a major public health concern requiring continuous efforts for understanding its epidemiology. Patients infected with SARS-CoV-2 have a wide range of clinical features ranging from asymptomatic infection to mild or severe illness with fatal outcomes or recovery. Population-based seroepidemiological studies are an effective method for measuring the rapid spread of SARS-CoV-2 and monitoring the pandemic's progress.
Methods: We conducted repeated cross-sectional community-based sentinel surveillance between January and June 2021 in the rural parts of the Pune district of Maharashtra, India to assess the seroprevalence against SARS-CoV-2 in three age categories. We selected 30 clusters for each round using a proportional population sampling method and 30 individuals in each of the three age groups (1-17 years, 18-49 years, and ≥50 years). We took blood samples from consenting study participants to check for the presence of Immunoglobulin G (IgG) antibodies against SARS-CoV-2 in all five rounds.
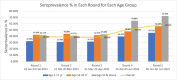
Results: We included 14 274 individuals across five rounds; 29% were from the 1-17, 39% from the 18-49, and 32% from the ≥50-year-old group. Overall seroprevalence combining all rounds was 45%. There was an increase in seropositivity in rounds four (51.15%) and five (58.32%) contributed mostly by adults. We found that about 72% of elderly individuals ≥50 years in round five were seropositive. The factors strongly associated with the seropositivity were being in contact with suspected or confirmed cases of COVID-19 (odds ratio (OR) = 7.15; 95% confidence interval (CI) = 4.2-12.14), receiving at least one dose of COVID-19 vaccine (OR = 3.13 (95% CI = 0.70-14.07), being aged ≥50 years (OR = 1.97; 95% CI = 1.81-2.15), and being in an occupation belonging to a high-risk category (OR = 1.92; 95% CI = 1.65-2.26). Among 135 hospitalizations reported due to COVID-19-like illness, 91 (67%) were in the elderly age group of ≥50 and 33 (24%) were in the 18-49-year-old age group.
Conclusions: Seroprevalence of SARS-CoV-2 was high in the last two rounds (April to June 2021) which coincide with the second wave of the pandemic (Delta variant B.1.617.2) in India. Overall, one in three children and one in two adults had antibodies for SARS-CoV-2. The suspected or confirmed case of COVID-19 emerged as the significant factor strongly associated with the seropositivity followed by COVID-19 vaccination.
Copyright © 2023 by the Journal of Global Health. All rights reserved.
Conflict of interest statement
Disclosure of interest: The authors completed the ICMJE Disclosure of Interest Form (available upon request from the corresponding author) and disclose the following activities and/or relationships: HN has received grant funding from Innovative Medicines Initiative, World Health Organisation, Bill and Melinda Gates Foundation, Pfizer and Icosavax. He has received honoraria from Sanofi, GSK, Merck, Novavax, Reviral. Other authors declare that they have no competing interests.
Figures
References
-
- John Hopkins University of Medicine. Johns Hopkins Coronavirus Resource Center. Available: https://coronavirus.jhu.edu/. Accessed: 31 August 2022.
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous