Overlap of Genetic Loci for Central Serous Chorioretinopathy With Age-Related Macular Degeneration
- PMID: 37079300
- PMCID: PMC10119776
- DOI: 10.1001/jamaophthalmol.2023.0706
Overlap of Genetic Loci for Central Serous Chorioretinopathy With Age-Related Macular Degeneration
Erratum in
-
Error in Author Affiliations.JAMA Ophthalmol. 2023 Jul 1;141(7):697. doi: 10.1001/jamaophthalmol.2023.2486. JAMA Ophthalmol. 2023. PMID: 37261820 Free PMC article. No abstract available.
Abstract
Importance: Central serous chorioretinopathy (CSC) is a serous maculopathy of unknown etiology. Two of 3 previously reported CSC genetic risk loci are also associated with AMD. Improved understanding of CSC genetics may broaden our understanding of this genetic overlap and unveil mechanisms in both diseases.
Objective: To identify novel genetic risk factors for CSC and compare genetic risk factors for CSC and AMD.
Design, setting, and participants: Using International Classification of Diseases, Ninth (ICD-9) and Tenth (ICD-10) Revision code-based inclusion and exclusion criteria, patients with CSC and controls were identified in both the FinnGen study and the Estonian Biobank (EstBB). Also included in a meta-analysis were previously reported patients with chronic CSC and controls. Data were analyzed from March 1 to September 31, 2022.
Main outcomes and measures: Genome-wide association studies (GWASs) were performed in the biobank-based cohorts followed by a meta-analysis of all cohorts. The expression of genes prioritized by the polygenic priority score and nearest-gene methods were assessed in cultured choroidal endothelial cells and public ocular single-cell RNA sequencing data sets. The predictive utility of polygenic scores (PGSs) for CSC and AMD were evaluated in the FinnGen study.
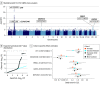
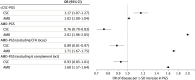
Results: A total of 1176 patients with CSC and 526 787 controls (312 162 female [59.3%]) were included in this analysis: 552 patients with CSC and 343 461 controls were identified in the FinnGen study, 103 patients with CSC and 178 573 controls were identified in the EstBB, and 521 patients with chronic CSC and 3577 controls were included in a meta-analysis. Two previously reported CSC risk loci were replicated (near CFH and GATA5) and 3 novel loci were identified (near CD34/46, NOTCH4, and PREX1). The CFH and NOTCH4 loci were associated with AMD but in the opposite direction. Prioritized genes showed increased expression in cultured choroidal endothelial cells compared with other genes in the loci (median [IQR] of log 2 [counts per million], 7.3 [0.6] vs 4.7 [3.7]; P = .004) and were differentially expressed in choroidal vascular endothelial cells in single-cell RNA sequencing data (mean [SD] fold change, 2.05 [0.38] compared with other cell types; P < 7.1 × 10-20). A PGS for AMD was predictive of reduced CSC risk (odds ratio, 0.76; 95% CI, 0.70-0.83 per +1 SD in AMD-PGS; P = 7.4 × 10-10). This association may have been mediated by loci containing complement genes.
Conclusions and relevance: In this 3-cohort genetic association study, 5 genetic risk loci for CSC were identified, highlighting a likely role for genes involved in choroidal vascular function and complement regulation. Results suggest that polygenic AMD risk was associated with reduced risk of CSC and that this genetic overlap was largely due to loci containing complement genes.
Conflict of interest statement
Figures


Comment in
-
What Can We Learn From the Surprising Insight Into the Genetic Background of Age-Related Macular Degeneration and Central Serous Chorioretinopathy?JAMA Ophthalmol. 2023 May 1;141(5):457-458. doi: 10.1001/jamaophthalmol.2023.0927. JAMA Ophthalmol. 2023. PMID: 37079325 No abstract available.
Similar articles
-
Genome-Wide Association Study of Age-Related Macular Degeneration Reveals 2 New Loci Implying Shared Genetic Components with Central Serous Chorioretinopathy.Ophthalmology. 2023 Apr;130(4):361-372. doi: 10.1016/j.ophtha.2022.10.034. Epub 2022 Nov 22. Ophthalmology. 2023. PMID: 36423732
-
Genome-wide Survival Analysis for Macular Neovascularization Development in Central Serous Chorioretinopathy Revealed Shared Genetic Susceptibility with Polypoidal Choroidal Vasculopathy.Ophthalmology. 2022 Sep;129(9):1034-1042. doi: 10.1016/j.ophtha.2022.04.018. Epub 2022 Apr 29. Ophthalmology. 2022. PMID: 35490733
-
Chronic central serous chorioretinopathy is associated with genetic variants implicated in age-related macular degeneration.Ophthalmology. 2015 Mar;122(3):562-70. doi: 10.1016/j.ophtha.2014.09.026. Epub 2014 Nov 6. Ophthalmology. 2015. PMID: 25439433
-
Morphologic features of large choroidal vessel layer: age-related macular degeneration, polypoidal choroidal vasculopathy, and central serous chorioretinopathy.Graefes Arch Clin Exp Ophthalmol. 2018 Dec;256(12):2309-2317. doi: 10.1007/s00417-018-4143-1. Epub 2018 Sep 27. Graefes Arch Clin Exp Ophthalmol. 2018. PMID: 30259090 Review.
-
Hypothetical pathogenesis of age-related macular degeneration and pachychoroid diseases derived from their genetic characteristics.Jpn J Ophthalmol. 2020 Nov;64(6):555-567. doi: 10.1007/s10384-020-00773-w. Epub 2020 Oct 2. Jpn J Ophthalmol. 2020. PMID: 33006732 Review.
Cited by
-
Shared whole environmental etiology between Alzheimer's disease and age-related macular degeneration.NPJ Aging. 2024 Aug 5;10(1):36. doi: 10.1038/s41514-024-00162-4. NPJ Aging. 2024. PMID: 39103390 Free PMC article.
-
Error in Author Affiliations.JAMA Ophthalmol. 2023 Jul 1;141(7):697. doi: 10.1001/jamaophthalmol.2023.2486. JAMA Ophthalmol. 2023. PMID: 37261820 Free PMC article. No abstract available.
-
Rare genetic variation in VE-PTP is associated with central serous chorioretinopathy, venous dysfunction and glaucoma.medRxiv [Preprint]. 2024 May 9:2024.05.08.24307013. doi: 10.1101/2024.05.08.24307013. medRxiv. 2024. Update in: Nat Commun. 2025 May 3;16(1):4127. doi: 10.1038/s41467-025-58686-6. PMID: 38766240 Free PMC article. Updated. Preprint.
-
Variations in Using Diagnosis Codes for Defining Age-Related Macular Degeneration Cohorts.Informatics (MDPI). 2024 Jun;11(2):28. doi: 10.3390/informatics11020028. Epub 2024 May 1. Informatics (MDPI). 2024. PMID: 40012991 Free PMC article.
-
Targeting the Tie-2 Receptor With Faricimab in Central Serous Chorioretinopathy: A Case Series Motivated by a Genetic Finding.Am J Ophthalmol. 2025 Jan;269:246-254. doi: 10.1016/j.ajo.2024.08.040. Epub 2024 Sep 2. Am J Ophthalmol. 2025. PMID: 39233019 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

