Trends in Severe Outcomes Among Adult and Pediatric Patients Hospitalized With COVID-19 in the Canadian Nosocomial Infection Surveillance Program, March 2020 to May 2022
- PMID: 37079304
- PMCID: PMC10119741
- DOI: 10.1001/jamanetworkopen.2023.9050
Trends in Severe Outcomes Among Adult and Pediatric Patients Hospitalized With COVID-19 in the Canadian Nosocomial Infection Surveillance Program, March 2020 to May 2022
Erratum in
-
Error in Byline, Affiliations, and Contributions.JAMA Netw Open. 2023 May 1;6(5):e2317468. doi: 10.1001/jamanetworkopen.2023.17468. JAMA Netw Open. 2023. PMID: 37219910 Free PMC article. No abstract available.
-
Error in Methods.JAMA Netw Open. 2023 Aug 1;6(8):e2329475. doi: 10.1001/jamanetworkopen.2023.29475. JAMA Netw Open. 2023. PMID: 37552485 Free PMC article. No abstract available.
Abstract
Importance: Trends in COVID-19 severe outcomes have significant implications for the health care system and are key to informing public health measures. However, data summarizing trends in severe outcomes among patients hospitalized with COVID-19 in Canada are not well described.
Objective: To describe trends in severe outcomes among patients hospitalized with COVID-19 during the first 2 years of the COVID-19 pandemic.
Design, setting, and participants: Active prospective surveillance in this cohort study was conducted from March 15, 2020, to May 28, 2022, at a sentinel network of 155 acute care hospitals across Canada. Participants included adult (aged ≥18 years) and pediatric (aged 0-17 years) patients hospitalized with laboratory-confirmed COVID-19 at a Canadian Nosocomial Infection Surveillance Program (CNISP)-participating hospital.
Exposures: COVID-19 waves, COVID-19 vaccination status, and age group.
Main outcomes and measures: The CNISP collected weekly aggregate data on the following severe outcomes: hospitalization, admission to an intensive care unit (ICU), receipt of mechanical ventilation, receipt of extracorporeal membrane oxygenation, and all-cause in-hospital death.
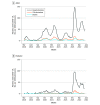
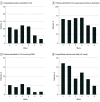
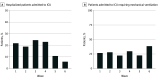
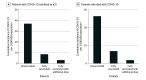
Results: Among 1 513 065 admissions, the proportion of adult (n = 51 679) and pediatric (n = 4035) patients hospitalized with laboratory-confirmed COVID-19 was highest in waves 5 and 6 of the pandemic compared with waves 1 to 4 (77.3 vs 24.7 per 1000 patient admissions). Despite this, the proportion of patients with positive test results for COVID-19 who were admitted to an ICU, received mechanical ventilation, received extracorporeal membrane oxygenation, and died were each significantly lower in waves 5 and 6 when compared with waves 1 through 4. Admission to the ICU and in-hospital all-cause death rates were significantly higher among those who were unvaccinated against COVID-19 when compared with those who were fully vaccinated (incidence rate ratio, 4.3 and 3.9, respectively) or fully vaccinated with an additional dose (incidence rate ratio, 12.2 and 15.1, respectively).
Conclusions and relevance: The findings of this cohort study of patients hospitalized with laboratory-confirmed COVID-19 suggest that COVID-19 vaccination is important to reduce the burden on the Canadian health care system as well as severe outcomes associated with COVID-19.
Conflict of interest statement
Figures
Similar articles
-
Trends in characteristics, interventions, and outcomes of hospitalized patients with COVID-19 in Canada: a multicentre prospective cohort study.Can J Anaesth. 2024 Dec;71(12):1745-1754. doi: 10.1007/s12630-024-02826-x. Epub 2024 Sep 4. Can J Anaesth. 2024. PMID: 39231882 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Characteristics and outcomes of patients with COVID-19 admitted to hospital and intensive care in the first phase of the pandemic in Canada: a national cohort study.CMAJ Open. 2021 Mar 8;9(1):E181-E188. doi: 10.9778/cmajo.20200250. Print 2021 Jan-Mar. CMAJ Open. 2021. PMID: 33688026 Free PMC article.
-
Outcomes of Coronavirus Disease 2019 Infection in Children and Adolescents With Cancer in Canada: Population-based Study and Systematic Review.J Pediatr Hematol Oncol. 2023 Aug 1;45(6):e689-e694. doi: 10.1097/MPH.0000000000002644. Epub 2023 Feb 20. J Pediatr Hematol Oncol. 2023. PMID: 36897636
-
A survey on Canadian pediatric hospital clinical/medical teaching unit implementation during the first and second wave of the COVID-19 pandemic.BMC Med Educ. 2021 Nov 11;21(1):570. doi: 10.1186/s12909-021-02994-0. BMC Med Educ. 2021. PMID: 34758814 Free PMC article. Review.
Cited by
-
Pediatric antibody responses to SARS-CoV-2 after infection and vaccination in Calgary, Canada.BMC Infect Dis. 2024 Jul 18;24(1):705. doi: 10.1186/s12879-024-09615-3. BMC Infect Dis. 2024. PMID: 39026179 Free PMC article.
-
Error in Methods.JAMA Netw Open. 2023 Aug 1;6(8):e2329475. doi: 10.1001/jamanetworkopen.2023.29475. JAMA Netw Open. 2023. PMID: 37552485 Free PMC article. No abstract available.
-
Impact of Pre-Existing Disability on Long-Term Health Care Use Following Hospitalization for COVID-19: A Population-Based Cohort Study.J Gen Intern Med. 2025 Feb 25. doi: 10.1007/s11606-025-09396-8. Online ahead of print. J Gen Intern Med. 2025. PMID: 40000520
-
Digital Contact Tracing Implementation Among Leaders and Health Care Workers in a Pediatric Hospital During the COVID-19 Pandemic: Qualitative Interview Study.JMIR Public Health Surveill. 2024 Nov 5;10:e64270. doi: 10.2196/64270. JMIR Public Health Surveill. 2024. PMID: 39499919 Free PMC article.
-
COVID-19 in Pediatric Patients With Acute Lymphoblastic Leukemia or Lymphoma.JAMA Netw Open. 2024 Feb 5;7(2):e2355727. doi: 10.1001/jamanetworkopen.2023.55727. JAMA Netw Open. 2024. PMID: 38363571 Free PMC article.
References
-
- Public Health Agency of Canada . COVID-19 signs, symptoms and severity of disease: a clinician guide. Updated June 1, 2022. Accessed March 23, 2022. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coro...
-
- Danza P, Koo TH, Haddix M, et al. . SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B.1.1.529 (Omicron) variant predominance—Los Angeles County, California, November 7, 2021–January 8, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(5):177-181. doi:10.15585/mmwr.mm7105e1 - DOI - PMC - PubMed
-
- Iuliano AD, Brunkard JM, Boehmer TK, et al. . Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods—United States, December 2020–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. doi:10.15585/mmwr.mm7104e4 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention . Science brief: SARS-CoV-2 infection-induced and vaccine-induced immunity. Updated October 29, 2021. Accessed May 26, 2022. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/vaccine... - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

