Evaluation of Plasma Biomarkers for A/T/N Classification of Alzheimer Disease Among Adults of Caribbean Hispanic Ethnicity
- PMID: 37079306
- PMCID: PMC10119732
- DOI: 10.1001/jamanetworkopen.2023.8214
Evaluation of Plasma Biomarkers for A/T/N Classification of Alzheimer Disease Among Adults of Caribbean Hispanic Ethnicity
Abstract
Importance: Cerebrospinal fluid (CSF) and plasma biomarkers can detect biological evidence of Alzheimer disease (AD), but their use in low-resource environments and among minority ethnic groups is limited.
Objective: To assess validated plasma biomarkers for AD among adults of Caribbean Hispanic ethnicity.
Design, setting, and participants: In this decision analytical modeling study, adults were recruited between January 1, 2018, and April 30, 2022, and underwent detailed clinical assessments and venipuncture. A subsample of participants also consented to lumbar puncture. Established CSF cut points were used to define AD biomarker-positive status, allowing determination of optimal cut points for plasma biomarkers in the same individuals. The performance of a panel of 6 plasma biomarkers was then assessed with respect to the entire group. Data analysis was performed in January 2023.
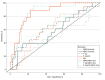
Main outcomes and measures: Main outcomes were the association of plasma biomarkers amyloid-β 1-42 (Aβ42), amyloid-β 1-40 (Aβ40), total tau (T-tau), phosphorylated tau181 (P-tau181), glial fibrillary acidic protein (GFAP), and neurofilament light chain (NfL) with AD diagnosis. These biomarkers allow assessment of amyloid (A), neurofibrillary degeneration (T), and neurodegeneration (N) aspects of AD. Statistical analyses performed included receiver operating characteristics, Pearson and Spearman correlations, t tests, and Wilcoxon rank-sum, chi-square, and Fisher exact tests.
Exposures: Exposures included age, sex, education, country of residence, apolipoprotein-ε4 (APOE-ε4) allele number, serum creatinine, blood urea nitrogen, and body mass index.
Results: This study included 746 adults. Participants had a mean (SD) age of 71.0 (7.8) years, 480 (64.3%) were women, and 154 (20.6%) met clinical criteria for AD. Associations were observed between CSF and plasma P-tau181 (r = .47 [95% CI, 0.32-0.60]), NfL (r = 0.57 [95% CI, 0.44-0.68]), and P-tau181/Aβ42 (r = 0.44 [95% CI, 0.29-0.58]). For AD defined by CSF biomarkers, plasma P-tau181 and P-tau181/Aβ42 provided biological evidence of AD. Among individuals judged to be clinically healthy without dementia, biomarker-positive status was determined by plasma P-tau181 for 133 (22.7%) and by plasma P-tau181/Aβ42 for 104 (17.7%). Among individuals with clinically diagnosed AD, 69 (45.4%) had plasma P-tau181 levels and 89 (58.9%) had P-tau181/Aβ42 levels that were inconsistent with AD. Individuals with biomarker-negative clinical AD status tended to have lower levels of education, were less likely to carry APOE-ε4 alleles, and had lower levels of GFAP and NfL than individuals with biomarker-positive clinical AD.
Conclusions and relevance: In this cross-sectional study, plasma P-tau181 and P-tau181/Aβ42 measurements correctly classified Caribbean Hispanic individuals with and without AD. However, plasma biomarkers identified individuals without dementia with biological evidence of AD, and a portion of those with dementia whose AD biomarker profile was negative. These results suggest that plasma biomarkers can augment detection of preclinical AD among asymptomatic individuals and improve the specificity of AD diagnosis.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

