Stereotactic Radiosurgery vs Conventional Radiotherapy for Localized Vertebral Metastases of the Spine: Phase 3 Results of NRG Oncology/RTOG 0631 Randomized Clinical Trial
- PMID: 37079324
- PMCID: PMC10119775
- DOI: 10.1001/jamaoncol.2023.0356
Stereotactic Radiosurgery vs Conventional Radiotherapy for Localized Vertebral Metastases of the Spine: Phase 3 Results of NRG Oncology/RTOG 0631 Randomized Clinical Trial
Abstract
Importance: Spine metastasis can be treated with high-dose radiation therapy with advanced delivery technology for long-term tumor and pain control.
Objective: To assess whether patient-reported pain relief was improved with stereotactic radiosurgery (SRS) as compared with conventional external beam radiotherapy (cEBRT) for patients with 1 to 3 sites of vertebral metastases.
Design, setting, and participants: In this randomized clinical trial, patients with 1 to 3 vertebral metastases were randomized 2:1 to the SRS or cEBRT groups. This NRG 0631 phase 3 study was performed as multi-institutional enrollment within NRG Oncology. Eligibility criteria included the following: (1) solitary vertebral metastasis, (2) 2 contiguous vertebral levels involved, or (3) maximum of 3 separate sites. Each site may involve up to 2 contiguous vertebral bodies. A total of 353 patients enrolled in the trial, and 339 patients were analyzed. This analysis includes data extracted on March 9, 2020.
Interventions: Patients randomized to the SRS group were treated with a single dose of 16 or 18 Gy (to convert to rad, multiply by 100) given to the involved vertebral level(s) only, not including any additional spine levels. Patients assigned to cEBRT were treated with 8 Gy given to the involved vertebra plus 1 additional vertebra above and below.
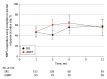
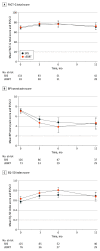
Main outcomes and measures: The primary end point was patient-reported pain response defined as at least a 3-point improvement on the Numerical Rating Pain Scale (NRPS) without worsening in pain at the secondary site(s) or the use of pain medication. Secondary end points included treatment-related toxic effects, quality of life, and long-term effects on vertebral bone and spinal cord.
Results: A total of 339 patients (mean [SD] age of SRS group vs cEBRT group, respectively, 61.9 [13.1] years vs 63.7 [11.9] years; 114 [54.5%] male in SRS group vs 70 [53.8%] male in cEBRT group) were analyzed. The baseline mean (SD) pain score at the index vertebra was 6.06 (2.61) in the SRS group and 5.88 (2.41) in the cEBRT group. The primary end point of pain response at 3 months favored cEBRT (41.3% for SRS vs 60.5% for cEBRT; difference, -19 percentage points; 95% CI, -32.9 to -5.5; 1-sided P = .99; 2-sided P = .01). Zubrod score (a measure of performance status ranging from 0 to 4, with 0 being fully functional and asymptomatic, and 4 being bedridden) was the significant factor influencing pain response. There were no differences in the proportion of acute or late adverse effects. Vertebral compression fracture at 24 months was 19.5% with SRS and 21.6% with cEBRT (P = .59). There were no spinal cord complications reported at 24 months.
Conclusions and relevance: In this randomized clinical trial, superiority of SRS for the primary end point of patient-reported pain response at 3 months was not found, and there were no spinal cord complications at 2 years after SRS. This finding may inform further investigation of using spine radiosurgery in the setting of oligometastases, where durability of cancer control is essential.
Trial registration: ClinicalTrials.gov Identifier: NCT00922974.
Conflict of interest statement
Figures

Comment in
-
Stereotactic Radiosurgery vs Conventional Radiotherapy for Spine Metastases.JAMA Oncol. 2024 Feb 1;10(2):259. doi: 10.1001/jamaoncol.2023.6077. JAMA Oncol. 2024. PMID: 38127328 No abstract available.
-
Stereotactic Radiosurgery vs Conventional Radiotherapy for Spine Metastases.JAMA Oncol. 2024 Feb 1;10(2):258-259. doi: 10.1001/jamaoncol.2023.6060. JAMA Oncol. 2024. PMID: 38127332 No abstract available.

