Transcription factor HNF4α2 promotes osteogenesis and prevents bone abnormalities in mice with renal osteodystrophy
- PMID: 37079387
- PMCID: PMC10231994
- DOI: 10.1172/JCI159928
Transcription factor HNF4α2 promotes osteogenesis and prevents bone abnormalities in mice with renal osteodystrophy
Abstract
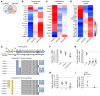
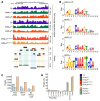
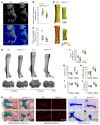
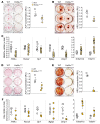
Renal osteodystrophy (ROD) is a disorder of bone metabolism that affects virtually all patients with chronic kidney disease (CKD) and is associated with adverse clinical outcomes including fractures, cardiovascular events, and death. In this study, we showed that hepatocyte nuclear factor 4α (HNF4α), a transcription factor mostly expressed in the liver, is also expressed in bone, and that osseous HNF4α expression was dramatically reduced in patients and mice with ROD. Osteoblast-specific deletion of Hnf4α resulted in impaired osteogenesis in cells and mice. Using multi-omics analyses of bones and cells lacking or overexpressing Hnf4α1 and Hnf4α2, we showed that HNF4α2 is the main osseous Hnf4α isoform that regulates osteogenesis, cell metabolism, and cell death. As a result, osteoblast-specific overexpression of Hnf4α2 prevented bone loss in mice with CKD. Our results showed that HNF4α2 is a transcriptional regulator of osteogenesis, implicated in the development of ROD.
Keywords: Bone Biology; Bone disease; Chronic kidney disease; Metabolism.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

