Concurrent HPV DNA testing and a visual inspection method for cervical precancer screening: A practical approach from Battor, Ghana
- PMID: 37079498
- PMCID: PMC10118129
- DOI: 10.1371/journal.pgph.0001830
Concurrent HPV DNA testing and a visual inspection method for cervical precancer screening: A practical approach from Battor, Ghana
Abstract
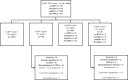
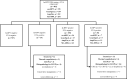
Cytology-based cervical cancer screening programs have been difficult to implement and scale up in developing countries. Thus, the World Health Organization recommends a 'see and treat' approach by way of hr-HPV testing and visual inspection. We aimed to evaluate concurrent HPV DNA testing and visual inspection in a real-world low-resource setting by comparing the detection rates of concurrent visual inspection with dilute acetic acid (VIA) or mobile colposcopy and hr-HPV DNA testing to standalone hr-HPV DNA testing (using the careHPV, GeneXpert, AmpFire, or MA-6000 platforms). We further compared their rates of loss to follow-up. This retrospective, descriptive cross-sectional study included all 4482 women subjected to cervical precancer screening at our facility between June 2016 and March 2022. The rates of EVA and VIA 'positivity' were 8.6% (95% CI, 6.7-10.6) and 2.1 (95% CI, 1.6-2.5), respectively, while the hr-HPV-positivity rate was 17.9% (95% CI, 16.7-19.0). Overall, 51 women in the entire cohort tested positive on both hr-HPV DNA testing and visual inspection (1.1%; 95% CI, 0.9-1.5), whereas a large majority of the women tested negative (3588/4482, 80.1%) for both and 2.1% (95% CI, 1.7-2.6) tested hr-HPV-negative but visual inspection 'positive'. In total, 191/275 (69.5%) participants who tested hr-HPV positive on any platform, as a standalone test for screening, returned for at least one follow-up visit. In light of factors such as poor socioeconomic circumstances, additional transportation costs associated with multiple screening visits, and lack of a reliable address system in many parts of Ghana, we posit that standalone HPV DNA testing with recall of hr-HPV positives will be tedious for a national cervical cancer prevention program. Our preliminary data show that concurrent testing (hr-HPV DNA testing alongside visual inspection by way of VIA or mobile colposcopy) may be more cost-effective than recalling hr-HPV-positive women for colposcopy.
Copyright: © 2023 Effah et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
LinkOut - more resources
Full Text Sources
