Direct Radical Copolymerizations of Thioamides To Generate Vinyl Polymers with Degradable Thioether Bonds in the Backbones
- PMID: 37079587
- PMCID: PMC10214439
- DOI: 10.1021/jacs.3c01796
Direct Radical Copolymerizations of Thioamides To Generate Vinyl Polymers with Degradable Thioether Bonds in the Backbones
Abstract
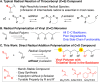
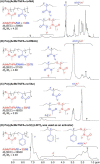
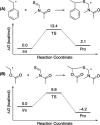
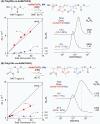
Direct radical addition reactions of thiocarbonyl (C═S) groups unaccompanied by β-scission have rarely been reported despite their potential for constructing various sulfur-containing compounds. Herein, we report direct radical copolymerizations of the C═S double bonds of simple thioamide derivatives and the C═C double bonds of common vinyl monomers to produce novel degradable vinyl polymers that contain thioether units in the backbones. In particular, N-acylated thioformamides copolymerized smoothly with various vinyl monomers, such as methyl acrylate, vinyl acetate, N,N-dimethylacrylamide, and styrene. RAFT copolymerization was also successfully mediated. The resultant copolymers had high glass transition temperatures and were readily degradable under ambient conditions. This work will expand the potential for use of thiocarbonyl compounds in radical reactions and develop novel poly(thioether)-vinyl polymer hybrid materials with unusual properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Crich D.; Quintero L. Radical Chemistry Associated with the Thiocarbonyl Group. Chem. Rev. 1989, 89, 1413–1432. 10.1021/cr00097a001. - DOI
-
- Moad G.; Rizzardo E.; Thang S. H. Radical Addition-Fragmentation Chemistry in Polymer Synthesis. Polymer 2008, 49, 1079–1131. 10.1016/j.polymer.2007.11.020. - DOI
-
- Bingham N. M.; Abousalman-Rezvani Z.; Collins K.; Roth P. J. Thiocarbonyl Chemistry in Polymer Science. Polym. Chem. 2022, 13, 2880–2901. 10.1039/D2PY00050D. - DOI
-
- Quiclet-Sire B.; Zard S. Z. Fun with Radicals: Some New Perspectives for Organic Synthesis. Pure Appl. Chem. 2010, 83, 519–551. 10.1351/PAC-CON-10-08-07. - DOI
-
- Tsuchihashi G.; Yamauchi M.; Ohno A. Thiocarbonyl Compounds as Potential Scavengers of Carbon Radicals. Bull. Chem. Soc. Jpn. 1970, 43, 968–968. 10.1246/bcsj.43.968. - DOI
LinkOut - more resources
Full Text Sources

