Explainable classification of Parkinson's disease using deep learning trained on a large multi-center database of T1-weighted MRI datasets
- PMID: 37079936
- PMCID: PMC10148079
- DOI: 10.1016/j.nicl.2023.103405
Explainable classification of Parkinson's disease using deep learning trained on a large multi-center database of T1-weighted MRI datasets
Abstract
Introduction: Parkinson's disease (PD) is a severe neurodegenerative disease that affects millions of people. Early diagnosis is important to facilitate prompt interventions to slow down disease progression. However, accurate PD diagnosis can be challenging, especially in the early disease stages. The aim of this work was to develop and evaluate a robust explainable deep learning model for PD classification trained from one of the largest collections of T1-weighted magnetic resonance imaging datasets.
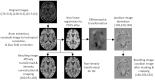
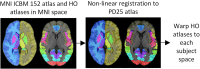
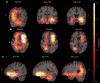
Materials and methods: A total of 2,041 T1-weighted MRI datasets from 13 different studies were collected, including 1,024 datasets from PD patients and 1,017 datasets from age- and sex-matched healthy controls (HC). The datasets were skull stripped, resampled to isotropic resolution, bias field corrected, and non-linearly registered to the MNI PD25 atlas. The Jacobian maps derived from the deformation fields together with basic clinical parameters were used to train a state-of-the-art convolutional neural network (CNN) to classify PD and HC subjects. Saliency maps were generated to display the brain regions contributing the most to the classification task as a means of explainable artificial intelligence.
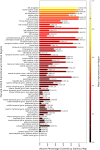
Results: The CNN model was trained using an 85%/5%/10% train/validation/test split stratified by diagnosis, sex, and study. The model achieved an accuracy of 79.3%, precision of 80.2%, specificity of 81.3%, sensitivity of 77.7%, and AUC-ROC of 0.87 on the test set while performing similarly on an independent test set. Saliency maps computed for the test set data highlighted frontotemporal regions, the orbital-frontal cortex, and multiple deep gray matter structures as most important.
Conclusion: The developed CNN model, trained on a large heterogenous database, was able to differentiate PD patients from HC subjects with high accuracy with clinically feasible classification explanations. Future research should aim to investigate the combination of multiple imaging modalities with deep learning and on validating these results in a prospective trial as a clinical decision support system.
Keywords: Deep learning; Explainable artificial intelligence; Magnetic Resonance Imaging; Parkinson’s disease.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

