Real-world comparative effectiveness of palbociclib plus letrozole versus letrozole in older patients with metastatic breast cancer
- PMID: 37080011
- PMCID: PMC10127113
- DOI: 10.1016/j.breast.2023.03.015
Real-world comparative effectiveness of palbociclib plus letrozole versus letrozole in older patients with metastatic breast cancer
Abstract
Background: Palbociclib, the first available cyclin-dependent kinase 4/6 inhibitor, plus endocrine therapy is approved for hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) metastatic breast cancer (MBC). This study compared real-world effectiveness of palbociclib plus letrozole versus letrozole in older patients with MBC in US clinical practice.
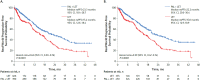
Methods: This retrospective analysis included patients from the Flatiron Health longitudinal database. Overall, 796 women with HR+/HER2- MBC aged ≥65 years starting palbociclib plus letrozole or letrozole as first-line therapy between February 2015 and September 2018 were included. Patients were evaluated from treatment start until December 2018, death, or last visit, whichever came first. Real-world progression-free survival (rwPFS), overall survival (OS), and real-world best tumor responses (rwBTR) were endpoints. Stabilized inverse probability treatment weighting (sIPTW) balanced patient characteristics.
Results: After sIPTW, 450 patients treated with palbociclib plus letrozole and 335 treated with letrozole were included; median age was 74.0 years. Median rwPFS was 22.2 (95% CI, 20.0-30.4) months for palbociclib plus letrozole versus 15.8 (12.9-18.9) months for letrozole (hazard ratio, 0.59 [0.47-0.74]; P<0.001). Median OS was not reached for palbociclib plus letrozole versus 43.4 months (30.0-not estimable) with letrozole (hazard ratio, 0.55 [0.42-0.72]; P<0.001). No interactions between age groups (65-74 and ≥75 years) and treatment groups were observed for rwPFS or OS. Rate of rwBTR was significantly higher for palbociclib plus letrozole (52.4%) versus letrozole (22.1%; odds ratio, 2.0 [1.4-2.7]; P<0.001).
Conclusion: This analysis demonstrates the effectiveness of palbociclib combination therapy as standard-of-care for older patients with HR+/HER2- MBC in the first-line setting.
Keywords: Letrozole; Metastatic breast cancer; Palbociclib; Real-world.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest Hope S. Rugo reports sponsored research to her institution from Pfizer Inc, Merck, Novartis, Eli Lilly, Roche, Daiichi-Sankyo, Seattle Genetics, Macrogenics, Sermonix, Boehringer Ingelheim, Polyphor, AstraZeneca, Ayala, and Gilead and honoraria from PUMA, Samsung, and Mylan. Rachel M. Layman has received research funding to her institution from Pfizer Inc, Eli Lilly, Novartis, GlaxoSmithKline, Puma, Zentalis, and Celcuity; and advisory board/consulting fees from Eli Lilly, Novartis, and Celcuity. Adam Brufsky has received consulting fees from Pfizer Inc, Lilly, Novartis, Eisai, AstraZeneca, Roche, Daiichi, and Merck. Xianchen Liu, Benjamin Li, and Lynn McRoy are employees of and own stock in Pfizer Inc.
Figures


References
-
- National Cancer Institute. Cancer stat facts: female breast cancer subtypes. Surveillance, Epidemiology, and End Results Program.
-
- National Comprehensive Cancer Network . 2020. NCCN clinical practice guidelines in Oncology (NCCN Guidelines®) breast cancer version 5.https://seer.cancer.gov/statfacts/html/breast-subtypes.html 2020. Accessed 2023.
-
- IBRANCE® capsules (palbociclib) Pfizer Inc; New York, NY: 2019. Full prescribing information.
-
- Turner N.C., Ro J., Andre F., Loi S., Verma S., Iwata H., et al. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–219. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

