Triciribine attenuates pathological neovascularization and vascular permeability in a mouse model of proliferative retinopathy
- PMID: 37080089
- PMCID: PMC10208444
- DOI: 10.1016/j.biopha.2023.114714
Triciribine attenuates pathological neovascularization and vascular permeability in a mouse model of proliferative retinopathy
Abstract
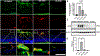
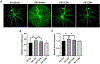
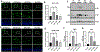
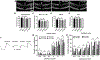
Proliferative retinopathies are the leading cause of irreversible blindness in all ages, and there is a critical need to identify novel therapies. We investigated the impact of triciribine (TCBN), a tricyclic nucleoside analog and a weak Akt inhibitor, on retinal neurovascular injury, vascular permeability, and inflammation in oxygen-induced retinopathy (OIR). Post-natal day 7 (P7) mouse pups were subjected to OIR, and treated (i.p.) with TCBN or vehicle from P14-P16 and compared with age-matched, normoxic, vehicle or TCBN-treated controls. P17 retinas were processed for flat mounts, immunostaining, Western blotting, and qRT-PCR studies. Fluorescein angiography, electroretinography, and spectral domain optical coherence tomography were performed on days P21, P26, and P30, respectively. TCBN treatment significantly reduced pathological neovascularization, vaso-obliteration, and inflammation marked by reduced TNFα, IL6, MCP-1, Iba1, and F4/80 (macrophage/microglia markers) expression compared to the vehicle-treated OIR mouse retinas. Pathological expression of VEGF (vascular endothelial growth factor), and claudin-5 compromised the blood-retinal barrier integrity in the OIR retinas correlating with increased vascular permeability and neovascular tuft formation, which were blunted by TCBN treatment. Of note, there were no changes in the retinal architecture or retinal cell function in response to TCBN in the normoxia or OIR mice. We conclude that TCBN protects against pathological neovascularization, restores blood-retinal barrier homeostasis, and reduces retinal inflammation without adversely affecting the retinal structure and neuronal function in a mouse model of OIR. Our data suggest that TCBN may provide a novel therapeutic option for proliferative retinopathy.
Keywords: Neovascular tufts; Neuroinflammation; Oxygen-induced retinopathy; Proliferative retinopathy; Triciribine; Vascular permeability.
Copyright © 2023 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Conflict of Interest Statement PRS is a scientific advisor of Ayma Therapeutics, NJ. All other authors declare that there are no financial or other conflicts of interest exist.
Figures






Similar articles
-
Epithelial Membrane Protein 2 (EMP2) Promotes VEGF-Induced Pathological Neovascularization in Murine Oxygen-Induced Retinopathy.Invest Ophthalmol Vis Sci. 2020 Feb 7;61(2):3. doi: 10.1167/iovs.61.2.3. Invest Ophthalmol Vis Sci. 2020. PMID: 32031575 Free PMC article.
-
Effect of Guibi-Tang, a Traditional Herbal Formula, on Retinal Neovascularization in a Mouse Model of Proliferative Retinopathy.Int J Mol Sci. 2015 Dec 16;16(12):29900-10. doi: 10.3390/ijms161226211. Int J Mol Sci. 2015. PMID: 26694358 Free PMC article.
-
T2-TrpRS inhibits preretinal neovascularization and enhances physiological vascular regrowth in OIR as assessed by a new method of quantification.Invest Ophthalmol Vis Sci. 2006 May;47(5):2125-34. doi: 10.1167/iovs.05-1096. Invest Ophthalmol Vis Sci. 2006. PMID: 16639024
-
Retinal vascular development and oxygen-induced retinopathy: a role for adenosine.Prog Retin Eye Res. 2003 Jan;22(1):95-111. doi: 10.1016/s1350-9462(02)00058-7. Prog Retin Eye Res. 2003. PMID: 12597925 Review.
-
[Pathological role of apelin in angiogenic eye disease].Yakugaku Zasshi. 2011;131(8):1201-6. doi: 10.1248/yakushi.131.1201. Yakugaku Zasshi. 2011. PMID: 21804324 Review. Japanese.
Cited by
-
Pro-Inflammatory Characteristics of Extracellular Vesicles in the Vitreous of Type 2 Diabetic Patients.Biomedicines. 2024 Sep 10;12(9):2053. doi: 10.3390/biomedicines12092053. Biomedicines. 2024. PMID: 39335566 Free PMC article.
-
Spermine oxidase inhibitor, MDL 72527, reduced neovascularization, vascular permeability, and acrolein-conjugated proteins in a mouse model of ischemic retinopathy.Tissue Barriers. 2025 Jan 2;13(1):2347070. doi: 10.1080/21688370.2024.2347070. Epub 2024 Apr 29. Tissue Barriers. 2025. PMID: 38682891 Free PMC article.
-
Stage-dependent proteomic alterations in aqueous humor of diabetic retinopathy patients based on data-independent acquisition and parallel reaction monitoring.J Transl Med. 2025 Apr 25;23(1):476. doi: 10.1186/s12967-025-06452-z. J Transl Med. 2025. PMID: 40281624 Free PMC article.
-
Pre-Existing Diabetes Alters Pulmonary Inflammatory Gene Expression Priming for Injury.FASEB J. 2025 Jul 31;39(14):e70804. doi: 10.1096/fj.202500816R. FASEB J. 2025. PMID: 40654181 Free PMC article.
-
Claudin-17 Deficiency Drives Vascular Permeability and Inflammation Causing Lung Injury.Int J Mol Sci. 2025 Apr 11;26(8):3612. doi: 10.3390/ijms26083612. Int J Mol Sci. 2025. PMID: 40332125 Free PMC article.
References
-
- Tonnies T, Brinks R, Isom S, Dabelea D, Divers J, Mayer-Davis EJ, Lawrence JM, Pihoker C, Dolan L, Liese AD, Saydah SH, D’Agostino RB, Hoyer A, Imperatore G, Projections of type 1 and type 2 diabetes burden in the U. S. population aged <20 years through 2060: the SEARCH for diabetes in youth study, Diabetes Care 46 (2) (2023) 313–320. - PMC - PubMed
-
- Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, Wong IY, Ting DSW, Tan GSW, Jonas JB, Sabanayagam C, Wong TY, Cheng CY, Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis, Ophthalmology 128 (11) (2021) 1580–1591. - PubMed
-
- Miloudi K, Oubaha M, Ménard C, Dejda A, Guber V, Cagnone G, Wilson AM, Tétreault N, Mawambo G, Binet F, Chidiac R, Delisle C, Buscarlet M, Cerani A, Crespo-Garcia S, Bentley K, Rezende F, Joyal JS, Mallette FA, Gratton JP, Larrivée A, Sapieha P, NOTCH1 signaling induces pathological vascular permeability in diabetic retinopathy, Proc. Natl. Acad. Sci. U. S. A 116 (10) (2019) 4538–4547. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

