Multicellular rosettes link mesenchymal-epithelial transition to radial intercalation in the mouse axial mesoderm
- PMID: 37080203
- PMCID: PMC10247533
- DOI: 10.1016/j.devcel.2023.03.018
Multicellular rosettes link mesenchymal-epithelial transition to radial intercalation in the mouse axial mesoderm
Abstract
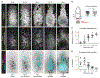
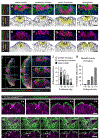
Mesenchymal-epithelial transitions are fundamental drivers of development and disease, but how these behaviors generate epithelial structure is not well understood. Here, we show that mesenchymal-epithelial transitions promote epithelial organization in the mouse node and notochordal plate through the assembly and radial intercalation of three-dimensional rosettes. Axial mesoderm rosettes acquire junctional and apical polarity, develop a central lumen, and dynamically expand, coalesce, and radially intercalate into the surface epithelium, converting mesenchymal-epithelial transitions into higher-order tissue structure. In mouse Par3 mutants, axial mesoderm rosettes establish central tight junction polarity but fail to form an expanded apical domain and lumen. These defects are associated with altered rosette dynamics, delayed radial intercalation, and formation of a small, fragmented surface epithelial structure. These results demonstrate that three-dimensional rosette behaviors translate mesenchymal-epithelial transitions into collective radial intercalation and epithelial formation, providing a strategy for building epithelial sheets from individual self-organizing units in the mammalian embryo.
Keywords: Par3; Pard3; axial mesoderm; embryo; mesenchymal-epithelial transition; morphogenesis; mouse; node; notochord; radial intercalation; rosette.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





Comment in
-
Rosettes guide the way for mesodermal MET.Dev Cell. 2023 Jun 5;58(11):917-918. doi: 10.1016/j.devcel.2023.04.018. Dev Cell. 2023. PMID: 37279697
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

