Data-driven adaptive needle insertion assist for transperineal prostate interventions
- PMID: 37080237
- PMCID: PMC10249778
- DOI: 10.1088/1361-6560/accefa
Data-driven adaptive needle insertion assist for transperineal prostate interventions
Abstract
Objective.Clinical outcomes of transperineal prostate interventions, such as biopsy, thermal ablations, and brachytherapy, depend on accurate needle placement for effectiveness. However, the accurate placement of a long needle, typically 150-200 mm in length, is challenging due to needle deviation induced by needle-tissue interaction. While several approaches for needle trajectory correction have been studied, many of them do not translate well to practical applications due to the use of specialized needles not yet approved for clinical use, or to relying on needle-tissue models that need to be tailored to individual patients.Approach.In this paper, we present a robot-assisted collaborative needle insertion method that only requires an actuated passive needle guide and a conventional needle. The method is designed to assist a physician inserting a needle manually through a needle guide. If the needle is deviated from the intended path, actuators shifts the needle radially in order to steer the needle trajectory and compensate for needle deviation adaptively. The needle guide is controlled by a new data-driven algorithm which does not requirea prioriinformation about needle or tissue properties. The method was evaluated in experiments with bothin vitroandex vivophantoms.Main results.The experiments inex vivotissue reported a mean final placement error of 0.36 mm with a reduction of 96.25% of placement error when compared to insertions without the use of assistive correction.Significance.Presented results show that the proposed closed-loop formulation can be successfully used to correct needle deflection during collaborative manual insertion with potential to be easily translated into clinical application.
Keywords: data-driven model; medical robotics; needle insertion assist; transperineal prostate intervention.
© 2023 Institute of Physics and Engineering in Medicine.
Figures

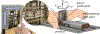








References
-
- Adagolodjo Y, Goffin L, De Mathelin M & Courtecuisse H (2019), ‘Robotic insertion of flexible needle in deformable structures using inverse finite-element simulation’, IEEE Transactions on Robotics 35(3).
-
- Alambeigi F, Wang Z, Hegeman R, Liu Y-H & Armand M (2018), ‘A robust data-driven approach for online learning and manipulation of unmodeled 3-D heterogeneous compliant objects’, IEEE Robotics and Automation Letters 3(4), 4140–4147.
-
- Baksic P, Courtecuisse H & Bayle B (2021), Shared control strategy for needle insertion into deformable tissue using inverse finite element simulation, in ‘Proc. IEEE International Conference on Robotics and Automation (ICRA)’, pp. 12442–12448.
-
- Bomers JG, Overduin CG, Jenniskens SF, Cornel EB, van Lin EN, Sedelaar JM & Fütterer JJ (2020), ‘Focal salvage MR imaging-guided cryoablation for localized prostate cancer recurrence after radiotherapy: 12-month follow-up’, Journal of Vascular and Interventional Radiology 31(1), 35–41. - PubMed
-
- Borot de Battisti M, Denis de Senneville B, Maenhout M, Lagendijk JJW, van Vulpen M, Hautvast G, Binnekamp D & Moerland MA (2016), ‘Fiber bragg gratings-based sensing for real-time needle tracking during MR-guided brachytherapy’, Medical Physics 43(10), 5288–5297. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
