Nickel nanoparticles induce autophagy and apoptosis via HIF-1α/mTOR signaling in human bronchial epithelial cells
- PMID: 37080518
- PMCID: PMC10231338
- DOI: 10.1016/j.envpol.2023.121670
Nickel nanoparticles induce autophagy and apoptosis via HIF-1α/mTOR signaling in human bronchial epithelial cells
Abstract
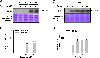
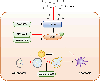
With the rapid development of nanotechnology, the potential adverse health effects of nanoparticles have been caught more attention and become global concerns. However, the underlying mechanisms in metal nanoparticle-induced toxic effects are still largely obscure. In this study, we investigated whether exposure to nickel nanoparticles (Nano-Ni) and titanium dioxide nanoparticles (Nano-TiO2) would alter autophagy and apoptosis levels in normal human bronchial epithelial BEAS-2B cells and the underlying mechanisms involved in this process. Our results showed that the expressions of autophagy- and apoptosis-associated proteins were dysregulated in cells exposed to Nano-Ni. However, exposure to the same doses of Nano-TiO2 had no significant effects on these proteins. In addition, exposure to Nano-Ni, but not Nano-TiO2, led to nuclear accumulation of HIF-1α and decreased phosphorylation of mTOR in BEAS-2B cells. Inhibition of HIF-1α by CAY10585 abolished Nano-Ni-induced decreased phosphorylation of mTOR, while activation of mTOR by MHY1485 did not affect Nano-Ni-induced nuclear accumulation of HIF-1α. Furthermore, both HIF-1α inhibition and mTOR activation abolished Nano-Ni-induced autophagy but enhanced Nano-Ni-induced apoptosis. Blockage of autophagic flux by Bafilomycin A1 exacerbated Nano-Ni-induced apoptosis, while activation of autophagy by Rapamycin effectively rescued Nano-Ni-induced apoptosis. In conclusion, our results demonstrated that Nano-Ni exposure caused increased levels of autophagy and apoptosis via the HIF-1α/mTOR signaling axis. Nano-Ni-induced autophagy has a protective role against Nano-Ni-induced apoptosis. These findings provide us with further insight into Nano-Ni-induced toxicity.
Keywords: Apoptosis; Autophagy; HIF-1α; Nickel nanoparticles; mTOR.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








Similar articles
-
Nickel nanoparticles induce epithelial-mesenchymal transition in human bronchial epithelial cells via the HIF-1α/HDAC3 pathway.Nanotoxicology. 2022 Aug-Oct;16(6-8):695-712. doi: 10.1080/17435390.2022.2142169. Epub 2022 Nov 7. Nanotoxicology. 2022. PMID: 36345150 Free PMC article.
-
Effects of metal nanoparticles on tight junction-associated proteins via HIF-1α/miR-29b/MMPs pathway in human epidermal keratinocytes.Part Fibre Toxicol. 2021 Mar 19;18(1):13. doi: 10.1186/s12989-021-00405-2. Part Fibre Toxicol. 2021. PMID: 33740985 Free PMC article.
-
Nickel nanoparticle-induced cell transformation: involvement of DNA damage and DNA repair defect through HIF-1α/miR-210/Rad52 pathway.J Nanobiotechnology. 2021 Nov 17;19(1):370. doi: 10.1186/s12951-021-01117-7. J Nanobiotechnology. 2021. PMID: 34789290 Free PMC article.
-
Toxicity assessment of metallic nickel nanoparticles in various biological models: An interplay of reactive oxygen species, oxidative stress, and apoptosis.Toxicol Ind Health. 2021 Oct;37(10):635-651. doi: 10.1177/07482337211011008. Epub 2021 Sep 7. Toxicol Ind Health. 2021. PMID: 34491146 Review.
-
Advance on toxicity of metal nickel nanoparticles.Environ Geochem Health. 2020 Jul;42(7):2277-2286. doi: 10.1007/s10653-019-00491-4. Epub 2020 Jan 1. Environ Geochem Health. 2020. PMID: 31894452 Review.
Cited by
-
Nickel-based nanomaterials: a comprehensive analysis of risk assessment, toxicity mechanisms, and future strategies for health risk prevention.J Nanobiotechnology. 2025 Mar 14;23(1):211. doi: 10.1186/s12951-025-03248-7. J Nanobiotechnology. 2025. PMID: 40087769 Free PMC article. Review.
-
The pulmonary effects of nickel-containing nanoparticles: Cytotoxicity, genotoxicity, carcinogenicity, and their underlying mechanisms.Environ Sci Nano. 2024 May 1;11(5):1817-1846. doi: 10.1039/d3en00929g. Epub 2024 Mar 21. Environ Sci Nano. 2024. PMID: 38984270 Free PMC article.
-
Nanomedicine Approaches for Autophagy Modulation in Cancer Therapy.Small Sci. 2025 Apr 11;5(6):2400607. doi: 10.1002/smsc.202400607. eCollection 2025 Jun. Small Sci. 2025. PMID: 40529859 Free PMC article.
-
Inhalation exposure-induced toxicity and disease mediated via mTOR dysregulation.Exp Biol Med (Maywood). 2024 Apr 22;249:10135. doi: 10.3389/ebm.2024.10135. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 38711460 Free PMC article. Review.
-
AFB1 Triggers Lipid Metabolism Disorders through the PI3K/Akt Pathway and Mediates Apoptosis Leading to Hepatotoxicity.Foods. 2024 Jan 3;13(1):163. doi: 10.3390/foods13010163. Foods. 2024. PMID: 38201191 Free PMC article.
References
-
- Bhattacharjee D, Sheet SK, Khatua S, Biswas K, Joshi S, Myrboh B, 2018. A reusable magnetic nickel nanoparticle based catalyst for the aqueous synthesis of diverse heterocycles and their evaluation as potential anti-bacterial agent. Bioorg Med Chem 26, 5018–5028. doi:10.1016/j.bmc.2018.08.033. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

