Creation of a structured molecular genomics report for Germany as a local adaption of HL7's Genomic Reporting Implementation Guide
- PMID: 37080557
- PMCID: PMC10198526
- DOI: 10.1093/jamia/ocad061
Creation of a structured molecular genomics report for Germany as a local adaption of HL7's Genomic Reporting Implementation Guide
Abstract
Objective: The objective was to develop a dataset definition, information model, and FHIR® specification for key data elements contained in a German molecular genomics (MolGen) report to facilitate genomic and phenotype integration in electronic health records.
Materials and methods: A dedicated expert group participating in the German Medical Informatics Initiative reviewed information contained in MolGen reports, determined the key elements, and formulated a dataset definition. HL7's Genomics Reporting Implementation Guide (IG) was adopted as a basis for the FHIR® specification which was subjected to a public ballot. In addition, elements in the MolGen dataset were mapped to the fields defined in ISO/TS 20428:2017 standard to evaluate compliance.
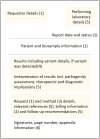
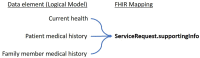
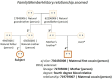
Results: A core dataset of 76 data elements, clustered into 6 categories was created to represent all key information of German MolGen reports. Based on this, a FHIR specification with 16 profiles, 14 derived from HL7®'s Genomics Reporting IG and 2 additional profiles (of the FamilyMemberHistory and RiskAssessment resources), was developed. Five example resource bundles show how our adaptation of an international standard can be used to model MolGen report data that was requested following oncological or rare disease indications. Furthermore, the map of the MolGen report data elements to the fields defined by the ISO/TC 20428:2017 standard, confirmed the presence of the majority of required fields.
Conclusions: Our report serves as a template for other research initiatives attempting to create a standard format for unstructured genomic report data. Use of standard formats facilitates integration of genomic data into electronic health records for clinical decision support.
Keywords: FHIR®; Fast Healthcare Interoperability Resources; HL7®; Implementation Guide; interoperability; molecular genomics report; sequencing data.
© The Author(s) 2023. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
ST is vice chair of HL7 Germany. The other authors declare no competing interests.
Figures


Similar articles
-
Clinical Genomic Sequencing Reports in Electronic Health Record Systems Based on International Standards: Implementation Study.J Med Internet Res. 2020 Aug 10;22(8):e15040. doi: 10.2196/15040. J Med Internet Res. 2020. PMID: 32773368 Free PMC article.
-
Interoperable genetic lab test reports: mapping key data elements to HL7 FHIR specifications and professional reporting guidelines.J Am Med Inform Assoc. 2021 Nov 25;28(12):2617-2625. doi: 10.1093/jamia/ocab201. J Am Med Inform Assoc. 2021. PMID: 34569596 Free PMC article.
-
Genomic considerations for FHIR®; eMERGE implementation lessons.J Biomed Inform. 2021 Jun;118:103795. doi: 10.1016/j.jbi.2021.103795. Epub 2021 Apr 28. J Biomed Inform. 2021. PMID: 33930535 Free PMC article.
-
Health Care in the Age of Interoperability Part 5: The Personal Health Record.IEEE Pulse. 2019 May-Jun;10(3):19-23. doi: 10.1109/MPULS.2019.2911804. IEEE Pulse. 2019. PMID: 31135347 Review.
-
Learning HL7 FHIR Using the HAPI FHIR Server and Its Use in Medical Imaging with the SIIM Dataset.J Digit Imaging. 2018 Jun;31(3):334-340. doi: 10.1007/s10278-018-0090-y. J Digit Imaging. 2018. PMID: 29725959 Free PMC article. Review.
Cited by
-
Empowering personalized oncology: evolution of digital support and visualization tools for molecular tumor boards.BMC Med Inform Decis Mak. 2025 Jan 16;25(1):29. doi: 10.1186/s12911-024-02821-8. BMC Med Inform Decis Mak. 2025. PMID: 39819625 Free PMC article.
-
An ontology-based rare disease common data model harmonising international registries, FHIR, and Phenopackets.Sci Data. 2025 Feb 8;12(1):234. doi: 10.1038/s41597-025-04558-z. Sci Data. 2025. PMID: 39922817 Free PMC article.
-
[Interoperability Working Group: core dataset and information systems for data integration and data exchange in the Medical Informatics Initiative].Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2024 Jun;67(6):656-667. doi: 10.1007/s00103-024-03888-4. Epub 2024 May 16. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2024. PMID: 38753022 Free PMC article. German.
-
Exchange of Quantitative Computed Tomography Assessed Body Composition Data Using Fast Healthcare Interoperability Resources as a Necessary Step Toward Interoperable Integration of Opportunistic Screening Into Clinical Practice: Methodological Development Study.J Med Internet Res. 2025 May 21;27:e68750. doi: 10.2196/68750. J Med Internet Res. 2025. PMID: 40397929 Free PMC article.
-
Genomics on FHIR - a feasibility study to support a National Strategy for Genomic Medicine.NPJ Genom Med. 2025 Jul 29;10(1):57. doi: 10.1038/s41525-025-00516-1. NPJ Genom Med. 2025. PMID: 40730790 Free PMC article.
References
-
- National Cancer Institute. Biomarker Testing for Cancer Treatment. 2017. https://www.cancer.gov/about-cancer/treatment/types/biomarker-testing-ca.... Accessed December 16, 2022.

