Long-term inhaled treprostinil for pulmonary hypertension due to interstitial lung disease: INCREASE open-label extension study
- PMID: 37080567
- PMCID: PMC10307984
- DOI: 10.1183/13993003.02414-2022
Long-term inhaled treprostinil for pulmonary hypertension due to interstitial lung disease: INCREASE open-label extension study
Abstract
Introduction: The 16-week randomised, placebo-controlled INCREASE trial (RCT) met its primary end-point by improving 6-min walk distance (6MWD) in patients receiving inhaled treprostinil for pulmonary hypertension due to interstitial lung disease (PH-ILD). The open-label extension (OLE) evaluated long-term effects of inhaled treprostinil in PH-ILD.
Methods: Of 258 eligible patients, 242 enrolled in the INCREASE OLE and received inhaled treprostinil. Assessments included 6MWD, pulmonary function testing, N-terminal pro-brain natriuretic peptide (NT-proBNP), quality of life and adverse events. Hospitalisations, exacerbations of underlying lung disease and death were recorded.
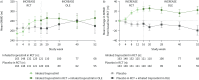
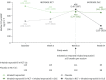
Results: At INCREASE OLE baseline, patients had a median age of 70 years and a mean 6MWD of 274.2 m; 52.1% were male. For the overall population, the mean 6MWD at week 52 was 279.1 m and the mean change from INCREASE RCT baseline was 3.5 m (22.1 m for the prior inhaled treprostinil arm and -19.5 m for the prior placebo arm); the median NT-proBNP decreased from 389 pg·mL-1 at RCT baseline to 359 pg·mL-1 at week 64; and the absolute (% predicted) mean forced vital capacity change from RCT baseline to week 64 was 51 mL (2.8%). Patients who received inhaled treprostinil versus placebo in the RCT had a 31% lower relative risk of exacerbation of underlying lung disease in the OLE (hazard ratio 0.69 (95% CI 0.49-0.97); p=0.03). Adverse events leading to drug discontinuation occurred in 54 (22.3%) patients.
Conclusions: These results support the long-term safety and efficacy of inhaled treprostinil in patients with PH-ILD, and are consistent with the results observed in the INCREASE RCT.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: A. Waxman reports that the present manuscript was funded by United Therapeutics; and reports grants or contracts with payments to his institution from United Therapeutics, Gossamer, Acceleron/Merck, ARIA CV and NIH/NHLBI, patents with Johnson & Johnson, and participation on a data safety monitoring board or advisory board for INSMED. R. Restrepo-Jaramillo reports research grants from Merck, United Therapeutics, Bayer and J&J Actelion, payment of honoraria for speakers bureaus from United Therapeutics, Bayer and J&J Actelion, and participation on advisory boards for Merck, United Therapeutics and Bayer. T. Thenappan reports grants for clinical research studies from United Therapeutics, Tenax Therapeutics, Aria CV and Merck, and consulting fees from United Therapeutics and Acceleron Pharma. P. Engel reports no disclosures. A. Bajwa reports a grant from United Therapeutics for study procedures, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from United Therapeutics, Bayer, Johnson & Johnson, Boehringer Ingelheim, and Intuitive. A. Ravichandran reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from United Therapeutics, Bayer, Abbott, Medtronic and Janssen. J. Feldman reports consulting fees from United Therapeutics, Aerovate, Altavant and Liquidia, payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Janssen and United Therapeutics, and participation on a data safety monitoring board or advisory board for Liquidia, Altavant and Aerovate. A. Hajari Case reports that the present manuscript is funded by United Therapeutics; and reports clinical research contracts to institution from United Therapeutics, and serving as Senior Medical Advisor for the Pulmonary Fibrosis Foundation. R.G. Argula reports consulting fees from Liquidia Corporation, United Therapeutics, Merck Pharma Inc., Janssen Inc. and CVS Health, and payment or honoraria for lectures and educational events from United Therapeutics. V. Tapson reports research grants paid to institution from Boston Scientific Corporation, Bristol Myers Squibb and Genentech, speaker and consulting fees from Janssen, fees from Boston Scientific Corporation for participating in an executive committee for a clinical trial, that he serves as President for PE Response Team Consortium (unpaid), owns stock in Thrombolex and Inari Medical, and that he is employed as VP of Medical Affairs at Inari Medical. P. Smith reports that he is an employee of the study sponsor, United Therapeutics. C. Deng and E. Shen report that they are employees of and hold stock in United Therapeutics, the study sponsor. S.D. Nathan reports that the present manuscript is funded by United Therapeutics; and reports consulting fees from United Therapeutics, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from United Therapeutics.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
