Age-related changes in plasma biomarkers and their association with mortality in COVID-19
- PMID: 37080568
- PMCID: PMC10151455
- DOI: 10.1183/13993003.00011-2023
Age-related changes in plasma biomarkers and their association with mortality in COVID-19
Erratum in
-
"Age-related changes in plasma biomarkers and their association with mortality in COVID-19." E.H.A. Michels, B. Appelman, J. de Brabander, et al. Eur Respir J 2023; 62: 2300011.Eur Respir J. 2024 Feb 29;63(2):2350011. doi: 10.1183/13993003.50011-2023. Print 2024 Feb. Eur Respir J. 2024. PMID: 38423593 Free PMC article. No abstract available.
Abstract
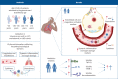
Background: Coronavirus disease 2019 (COVID-19)-induced mortality occurs predominantly in older patients. Several immunomodulating therapies seem less beneficial in these patients. The biological substrate behind these observations is unknown. The aim of this study was to obtain insight into the association between ageing, the host response and mortality in patients with COVID-19.
Methods: We determined 43 biomarkers reflective of alterations in four pathophysiological domains: endothelial cell and coagulation activation, inflammation and organ damage, and cytokine and chemokine release. We used mediation analysis to associate ageing-driven alterations in the host response with 30-day mortality. Biomarkers associated with both ageing and mortality were validated in an intensive care unit and external cohort.
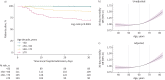
Results: 464 general ward patients with COVID-19 were stratified according to age decades. Increasing age was an independent risk factor for 30-day mortality. Ageing was associated with alterations in each of the host response domains, characterised by greater activation of the endothelium and coagulation system and stronger elevation of inflammation and organ damage markers, which was independent of an increase in age-related comorbidities. Soluble tumour necrosis factor receptor 1, soluble triggering receptor expressed on myeloid cells 1 and soluble thrombomodulin showed the strongest correlation with ageing and explained part of the ageing-driven increase in 30-day mortality (proportion mediated: 13.0%, 12.9% and 12.6%, respectively).
Conclusions: Ageing is associated with a strong and broad modification of the host response to COVID-19, and specific immune changes likely contribute to increased mortality in older patients. These results may provide insight into potential age-specific immunomodulatory targets in COVID-19.
Copyright ©The authors 2023.
Conflict of interest statement
Conflicts of interest: The authors declare no potential conflicts of interest.
Figures




Comment in
-
Age, host response, and mortality in COVID-19.Eur Respir J. 2023 Jul 7;62(1):2300796. doi: 10.1183/13993003.00796-2023. Print 2023 Jul. Eur Respir J. 2023. PMID: 37419522 Free PMC article.
References
-
- National Institute for Public Health and the Environment (RIVM) . COVID-19 deaths in the Netherlands by age. 2022. https://coronadashboard.government.nl/landelijk/sterfte Date last accessed: 22 April 2023.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
