A novel thiol-saccharide mucolytic for the treatment of muco-obstructive lung diseases
- PMID: 37080569
- PMCID: PMC10209473
- DOI: 10.1183/13993003.02022-2022
A novel thiol-saccharide mucolytic for the treatment of muco-obstructive lung diseases
Abstract
Background: Mucin disulfide cross-links mediate pathologic mucus formation in muco-obstructive lung diseases. MUC-031, a novel thiol-modified carbohydrate compound, cleaves disulfides to cause mucolysis. The aim of this study was to determine the mucolytic and therapeutic effects of MUC-031 in sputum from patients with cystic fibrosis (CF) and mice with muco-obstructive lung disease (βENaC-Tg mice).
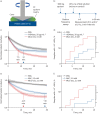
Methods: We compared the mucolytic efficacy of MUC-031 and existing mucolytics (N-acetylcysteine (NAC) and recombinant human deoxyribonuclease I (rhDNase)) using rheology to measure the elastic modulus (G') of CF sputum, and we tested effects of MUC-031 on airway mucus plugging, inflammation and survival in βENaC-Tg mice to determine its mucolytic efficacy in vivo.
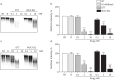
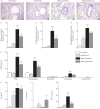
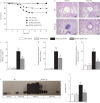
Results: In CF sputum, compared to the effects of rhDNase and NAC, MUC-031 caused a larger decrease in sputum G', was faster in decreasing sputum G' by 50% and caused mucolysis of a larger proportion of sputum samples within 15 min of drug addition. Compared to vehicle control, three treatments with MUC-031 in 1 day in adult βENaC-Tg mice decreased airway mucus content (16.8±3.2 versus 7.5±1.2 nL·mm-2, p<0.01) and bronchoalveolar lavage cells (73 833±6930 versus 47 679±7736 cells·mL-1, p<0.05). Twice-daily treatment with MUC-031 for 2 weeks also caused decreases in these outcomes in adult and neonatal βENaC-Tg mice and reduced mortality from 37% in vehicle-treated βENaC-Tg neonates to 21% in those treated with MUC-031 (p<0.05).
Conclusion: MUC-031 is a potent and fast-acting mucolytic that decreases airway mucus plugging, lessens airway inflammation and improves survival in βENaC-Tg mice. These data provide rationale for human trials of MUC-031 in muco-obstructive lung diseases.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: W. Raymond is co-inventor on a patent application (PCT/US19/50475) that describes MUC-031 and related structures. I. Gitlin reports support for the present work from NIH/NHLBI (P01HL128191), and is also co-inventor on a patent application (PCT/US19/50475) that describes MUC-031 and related structures, and a shareholder in Aer Therapeutics, an early-stage company that has licensed MUC-031 technology. A. Charbit reports support for the present work from NIH/NHLBI (P01HL128191). X. Orain reports support for the present work from NIH/NHLBI (P01HL128191). A.W. Scheffler reports the following support for the present manuscript: statistical consulting coordinated via UCSF Clinical and Translational Science Institute, funds provided by NIH P01HL128191. S.Y. Graeber reports grants from Mukoviszidose e.V. (German CF Foundation), Vertex Pharmaceuticals Incorporated, German Ministry for Education and Research (BMBF) and Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), and lecture honoraria from and advisory board participation for Chiesi GmbH and Vertex Pharmaceuticals Incorporated, outside the submitted work. A-M. Healy reports support for the present manuscript from NIH/NHLBI (P01HL128191, R01HL080414) and Science Foundation Ireland (SFI) (grants 12/RC/2275, 12/RC/2275_P2, 12/RC/2278), and is a shareholder in Aer Therapeutics, an early-stage company that has licensed MUC-031 technology. S. Oscarson reports support for the present manuscript from NIH/NHLBI (P01HL128191, R01HL080414), and is also co-inventor on a patent application (PCT/US19/50475) that describes MUC-031 and related structures, and a shareholder in Aer Therapeutics, an early-stage company that has licensed MUC-031 technology. J.V. Fahy reports grants from NIH/NHLBI, consulting fees from Suzhou Connect Biopharmaceuticals, Ltd, stock options/grants from Suzhou Connect Biopharmaceuticals, Ltd and Aer Therapeutics, outside the submitted work; and is also the inventor of patents PCT/US2014/028656 and PCT/US19/50475, which describe thiol-modified saccharides as drugs to treat mucus associated lung diseases. M.A. Mall reports support for the present manuscript from National Heart, Lung and Blood Institute (P01HL128191), German Ministry for Education and Research (BMBF) (82DZL009B1), Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) (CRC 1449 – 431232613 (A01, C04 and Z02)), and also reports, outside the present work, consulting fees from Boehringer Ingelheim, Arrowhead Pharmaceuticals, Vertex Pharmaceuticals, Santhera, Sterna Biologicals, Enterprise Therapeutics, Antabio and Abbvie, lecture honoraria from Boehringer Ingelheim, Arrowhead Pharmaceuticals and Vertex Pharmaceuticals, travel support from Boehringer Ingelheim and Vertex Pharmaceuticals, advisory board participation with Boehringer Ingelheim, Arrowhead Pharmaceuticals, Vertex Pharmaceuticals, Santhera, Enterprise Therapeutics, Antabio, Kither Biotech, Abbvie and Pari; and was a member of the board (2012–2020) and vice-president (2018–2020) of European Cystic Fibrosis Society. All other authors have nothing to disclose.
Figures

Comment in
-
Towards a better mucolytic.Eur Respir J. 2023 May 25;61(5):2300619. doi: 10.1183/13993003.00619-2023. Print 2023 May. Eur Respir J. 2023. PMID: 37230504 Free PMC article. No abstract available.
-
May Podcast: Cystic fibrosis.Eur Respir J. 2023 May 31;61(5):23E6105. doi: 10.1183/13993003.E6105-2023. Print 2023 May. Eur Respir J. 2023. PMID: 37257907 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
