Effects of a Small-Molecule Perforin Inhibitor in a Mouse Model of CD8 T Cell-Mediated Neuroinflammation
- PMID: 37080596
- PMCID: PMC10119812
- DOI: 10.1212/NXI.0000000000200117
Effects of a Small-Molecule Perforin Inhibitor in a Mouse Model of CD8 T Cell-Mediated Neuroinflammation
Abstract
Background and objectives: Alteration of the blood-brain barrier (BBB) at the interface between blood and CNS parenchyma is prominent in most neuroinflammatory diseases. In several neurologic diseases, including cerebral malaria and Susac syndrome, a CD8 T cell-mediated targeting of endothelial cells of the BBB (BBB-ECs) has been implicated in pathogenesis.
Methods: In this study, we used an experimental mouse model to evaluate the ability of a small-molecule perforin inhibitor to prevent neuroinflammation resulting from cytotoxic CD8 T cell-mediated damage of BBB-ECs.
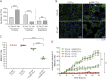
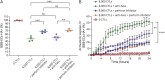
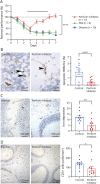
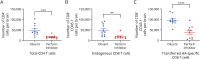
Results: Using an in vitro coculture system, we first identified perforin as an essential molecule for killing of BBB-ECs by CD8 T cells. We then found that short-term pharmacologic inhibition of perforin commencing after disease onset restored motor function and inhibited the neuropathology. Perforin inhibition resulted in preserved BBB-EC viability, maintenance of the BBB, and reduced CD8 T-cell accumulation in the brain and retina.
Discussion: Therefore, perforin-dependent cytotoxicity plays a key role in the death of BBB-ECs inflicted by autoreactive CD8 T cells in a preclinical model and potentially represents a therapeutic target for CD8 T cell-mediated neuroinflammatory diseases, such as cerebral malaria and Susac syndrome.
Written work prepared by employees of the Federal Government as part of their official duties is, under the U.S. Copyright Act, a “work of the United States Government” for which copyright protection under Title 17 of the United States Code is not available. As such, copyright does not extend to the contributions of employees of the Federal Government.
Conflict of interest statement
C. Fonte, E. Dufourd, V. Cazaentre, S. Aydin, B. Engelhardt, R. R. Caspi, B. Xu, G. Martin-Blondel, J.A. Spicer, and C. Bost report no disclosures relevant to the manuscript. C. Gonzalez-Fierro reports financial support by Fondation pour la Recherche Médicale (FRM). R.S. Liblau reports financial support by Fondation pour la Recherche Médicale (FRM), the French MS society (ARSEP foundation), Agence Nationale pour la Recherche CE17-0014, and BETPSY RHU 18- RHUS-0012. J. Bauer reports financial support by Austrian Science Fund FWF: P34864-B. J.A. Trapani reports financial support by the Strategic Drug Discovery Initiative (SDDI) Program of the Wellcome Trust UK. Go to
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
