Comparison of Nasal Swabs, Nasopharyngeal Swabs, and Saliva Samples for the Detection of SARS-CoV-2 and other Respiratory Virus Infections
- PMID: 37080744
- PMCID: PMC10151282
- DOI: 10.3343/alm.2023.43.5.434
Comparison of Nasal Swabs, Nasopharyngeal Swabs, and Saliva Samples for the Detection of SARS-CoV-2 and other Respiratory Virus Infections
Abstract
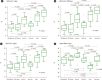
Background: Nasal swabs and saliva samples are being considered alternatives to nasopharyngeal swabs (NPSs) for detecting severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2); however, few studies have compared the usefulness of nasal swabs, NPSs, and saliva samples for detecting SARS-CoV-2 and other respiratory virus infections. We compared the positivity rates and concentrations of viruses detected in nasal swabs, NPSs, and saliva samples using cycle threshold (Ct) values from real-time PCR tests for respiratory viruses.
Methods: In total, 236 samples (48 five-rub and 10 10-rub nasal swabs, 96 NPSs collected using two different products, 48 saliva swabs, and 34 undiluted saliva samples) from 48 patients (34 patients with SARS-CoV-2 and 14 with other respiratory virus infections) and 40 samples from eight healthy controls were obtained. The PCR positivity and Ct values were compared using Allplex Respiratory Panels 1/2/3 and Allplex SARS-CoV-2 real-time PCR.
Results: NPSs showed the lowest Ct values (indicating the highest virus concentrations); however, nasal and saliva samples yielded positive results for SARS-CoV-2 and other respiratory viruses. The median Ct value for SARS-CoV-2 E gene PCR using nasal swab samples collected with 10 rubs was significantly different from that obtained using nasal swabs collected with five rubs (Ct=24.3 vs. 28.9; P=0.002), but not from that obtained using NPSs.
Conclusions: Our results confirm that the NPS is the best sample type for detecting respiratory viruses, but nasal swabs and saliva samples can be alternatives to NPSs. Vigorously and sufficiently rubbed nasal swabs can provide SARS-CoV-2 concentrations similar to those obtained with NPSs.
Keywords: Nasal; Nasopharynx; PCR; Respiratory virus; SARS-CoV-2; Saliva; Swab.
Conflict of interest statement
None declared.
Figures

Similar articles
-
Diagnostic Performance of Self-Collected Saliva Versus Nasopharyngeal Swab for the Molecular Detection of SARS-CoV-2 in the Clinical Setting.Microbiol Spectr. 2021 Dec 22;9(3):e0046821. doi: 10.1128/Spectrum.00468-21. Epub 2021 Nov 3. Microbiol Spectr. 2021. PMID: 34730436 Free PMC article.
-
Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: a systematic review and meta-analysis.Lancet Infect Dis. 2021 Sep;21(9):1233-1245. doi: 10.1016/S1473-3099(21)00146-8. Epub 2021 Apr 12. Lancet Infect Dis. 2021. PMID: 33857405 Free PMC article.
-
Direct comparison of clinical diagnostic sensitivity of saliva from buccal swabs versus combined oro-/nasopharyngeal swabs in the detection of SARS-CoV-2 B.1.1.529 Omicron.J Clin Virol. 2023 Aug;165:105496. doi: 10.1016/j.jcv.2023.105496. Epub 2023 May 24. J Clin Virol. 2023. PMID: 37269606 Free PMC article.
-
Adding saliva testing to oropharyngeal and deep nasal swab testing increases PCR detection of SARS-CoV-2 in primary care and children.Med J Aust. 2021 Sep 20;215(6):273-278. doi: 10.5694/mja2.51188. Epub 2021 Jul 20. Med J Aust. 2021. PMID: 34287935 Free PMC article.
-
Alternative clinical specimens for the detection of SARS-CoV-2: A rapid review.Rev Med Virol. 2021 Jul;31(4):e2185. doi: 10.1002/rmv.2185. Epub 2020 Oct 22. Rev Med Virol. 2021. PMID: 33091200 Review.
Cited by
-
Modified streptavidin-biotin based lateral flow test strip for rapid detection of SARS-CoV-2 S1 antigen in saliva samples.Sci Rep. 2024 Mar 27;14(1):7319. doi: 10.1038/s41598-024-57230-8. Sci Rep. 2024. PMID: 38538635 Free PMC article.
-
Impact of Swabbing Location, Self-Swabbing, and Food Intake on SARS-CoV-2 RNA Detection.Microorganisms. 2024 Mar 15;12(3):591. doi: 10.3390/microorganisms12030591. Microorganisms. 2024. PMID: 38543642 Free PMC article.
-
Specimen adequacy assay controls in nucleic acid amplification tests do not correlate with nasopharyngeal swab collection method.J Clin Microbiol. 2024 Oct 16;62(10):e0097524. doi: 10.1128/jcm.00975-24. Epub 2024 Sep 16. J Clin Microbiol. 2024. PMID: 39283073 Free PMC article. No abstract available.
-
Diagnostic performance, stability, and acceptability of self-collected saliva without additives for SARS-CoV-2 molecular diagnosis.Eur J Clin Microbiol Infect Dis. 2024 Jun;43(6):1127-1138. doi: 10.1007/s10096-024-04819-6. Epub 2024 Apr 13. Eur J Clin Microbiol Infect Dis. 2024. PMID: 38613706
-
At-home testing for respiratory viruses: a minireview of the current landscape.J Clin Microbiol. 2024 May 8;62(5):e0031223. doi: 10.1128/jcm.00312-23. Epub 2024 Mar 4. J Clin Microbiol. 2024. PMID: 38436246 Free PMC article. Review.
References
-
- Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis. 2018;67:e1–e94. doi: 10.1093/cid/ciy381. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

