CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy
- PMID: 37080958
- PMCID: PMC10119091
- DOI: 10.1038/s41467-023-37872-4
CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy
Abstract
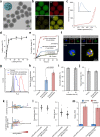
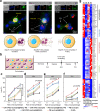
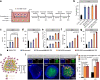
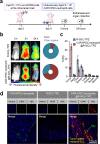
Glioblastoma (GBM) is one of the most aggressive and lethal solid tumors in human. While efficacious therapeutics, such as emerging chimeric antigen receptor (CAR)-T cells and chemotherapeutics, have been developed to treat various cancers, their effectiveness in GBM treatment has been hindered largely by the blood-brain barrier and blood-brain-tumor barriers. Human neutrophils effectively cross physiological barriers and display effector immunity against pathogens but the short lifespan and resistance to genome editing of primary neutrophils have limited their broad application in immunotherapy. Here we genetically engineer human pluripotent stem cells with CRISPR/Cas9-mediated gene knock-in to express various anti-GBM CAR constructs with T-specific CD3ζ or neutrophil-specific γ-signaling domains. CAR-neutrophils with the best anti-tumor activity are produced to specifically and noninvasively deliver and release tumor microenvironment-responsive nanodrugs to target GBM without the need to induce additional inflammation at the tumor sites. This combinatory chemo-immunotherapy exhibits superior and specific anti-GBM activities, reduces off-target drug delivery and prolongs lifespan in female tumor-bearing mice. Together, this biomimetic CAR-neutrophil drug delivery system is a safe, potent and versatile platform for treating GBM and possibly other devastating diseases.
© 2023. The Author(s).
Conflict of interest statement
Y.C., R.S., Q.D., and X.B. are inventors on two patent applications (human chimeric antigen receptor neutrophils, compositions, kits and methods of use (WO2022125850A1), and blood-brain barrier-penetrating CAR-neutrophil-mediated drug delivery (provisional patent)) for content described in this manuscript. X.B. is an advisory chief scientific officer (CSO) for Astheneia Bio. The remaining authors declare no other competing interests.
Figures


Similar articles
-
Current progress in chimeric antigen receptor T cell therapy for glioblastoma multiforme.Cancer Med. 2021 Aug;10(15):5019-5030. doi: 10.1002/cam4.4064. Epub 2021 Jun 19. Cancer Med. 2021. PMID: 34145792 Free PMC article. Review.
-
Local immunotherapy of glioblastoma: A comprehensive review of the concept.J Neuroimmunol. 2023 Aug 15;381:578146. doi: 10.1016/j.jneuroim.2023.578146. Epub 2023 Jul 7. J Neuroimmunol. 2023. PMID: 37451079 Review.
-
High-Density Lipoprotein-Mimicking Nanodiscs for Chemo-immunotherapy against Glioblastoma Multiforme.ACS Nano. 2019 Feb 26;13(2):1365-1384. doi: 10.1021/acsnano.8b06842. Epub 2019 Feb 11. ACS Nano. 2019. PMID: 30721028 Free PMC article.
-
Identification of genetic modifiers enhancing B7-H3-targeting CAR T cell therapy against glioblastoma through large-scale CRISPRi screening.J Exp Clin Cancer Res. 2024 Apr 1;43(1):95. doi: 10.1186/s13046-024-03027-6. J Exp Clin Cancer Res. 2024. PMID: 38561797 Free PMC article.
-
Prospective approaches to enhancing CAR T cell therapy for glioblastoma.Front Immunol. 2022 Oct 6;13:1008751. doi: 10.3389/fimmu.2022.1008751. eCollection 2022. Front Immunol. 2022. PMID: 36275671 Free PMC article. Review.
Cited by
-
Contemporary Approaches to Immunotherapy of Solid Tumors.Cancers (Basel). 2024 Jun 19;16(12):2270. doi: 10.3390/cancers16122270. Cancers (Basel). 2024. PMID: 38927974 Free PMC article. Review.
-
Biomimetic Nano-Drug Delivery System: An Emerging Platform for Promoting Tumor Treatment.Int J Nanomedicine. 2024 Jan 18;19:571-608. doi: 10.2147/IJN.S442877. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38260239 Free PMC article. Review.
-
Microenvironmental regulation of tumor-associated neutrophils in malignant glioma: from mechanism to therapy.J Neuroinflammation. 2024 Sep 16;21(1):226. doi: 10.1186/s12974-024-03222-4. J Neuroinflammation. 2024. PMID: 39285276 Free PMC article. Review.
-
CRISPR-Cas9-mediated homology-directed repair for precise gene editing.Mol Ther Nucleic Acids. 2024 Sep 26;35(4):102344. doi: 10.1016/j.omtn.2024.102344. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39494147 Free PMC article. Review.
-
Brain-targeting drug delivery systems: The state of the art in treatment of glioblastoma.Mater Today Bio. 2025 Jan 3;30:101443. doi: 10.1016/j.mtbio.2025.101443. eCollection 2025 Feb. Mater Today Bio. 2025. PMID: 39866779 Free PMC article. Review.
References
-
- Németh, T., Sperandio, M. & Mócsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov.10.1038/s41573-019-0054-z (2020). - PubMed
-
- Subhan, M. A. & Torchilin, V. P. Neutrophils as an emerging therapeutic target and tool for cancer therapy. Life Sci.10.1016/j.lfs.2021.119952 (2021). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

