Glucocorticoid activation of anti-inflammatory macrophages protects against insulin resistance
- PMID: 37080971
- PMCID: PMC10119112
- DOI: 10.1038/s41467-023-37831-z
Glucocorticoid activation of anti-inflammatory macrophages protects against insulin resistance
Abstract
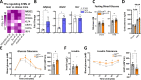
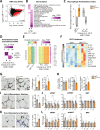
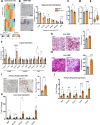
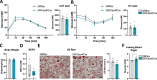
Insulin resistance (IR) during obesity is linked to adipose tissue macrophage (ATM)-driven inflammation of adipose tissue. Whether anti-inflammatory glucocorticoids (GCs) at physiological levels modulate IR is unclear. Here, we report that deletion of the GC receptor (GR) in myeloid cells, including macrophages in mice, aggravates obesity-related IR by enhancing adipose tissue inflammation due to decreased anti-inflammatory ATM leading to exaggerated adipose tissue lipolysis and severe hepatic steatosis. In contrast, GR deletion in Kupffer cells alone does not alter IR. Co-culture experiments show that the absence of GR in macrophages directly causes reduced phospho-AKT and glucose uptake in adipocytes, suggesting an important function of GR in ATM. GR-deficient macrophages are refractory to alternative ATM-inducing IL-4 signaling, due to reduced STAT6 chromatin loading and diminished anti-inflammatory enhancer activation. We demonstrate that GR has an important function in macrophages during obesity by limiting adipose tissue inflammation and lipolysis to promote insulin sensitivity.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Glucocorticoid activation of macrophages protects against insulin resistance.Nat Rev Endocrinol. 2023 Jul;19(7):380. doi: 10.1038/s41574-023-00850-3. Nat Rev Endocrinol. 2023. PMID: 37173438 No abstract available.
References
-
- Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

