Human CEACAM1 is targeted by a Streptococcus pyogenes adhesin implicated in puerperal sepsis pathogenesis
- PMID: 37080973
- PMCID: PMC10119177
- DOI: 10.1038/s41467-023-37732-1
Human CEACAM1 is targeted by a Streptococcus pyogenes adhesin implicated in puerperal sepsis pathogenesis
Erratum in
-
Author Correction: Human CEACAM1 is targeted by a Streptococcus pyogenes adhesin implicated in puerperal sepsis pathogenesis.Nat Commun. 2023 May 9;14(1):2675. doi: 10.1038/s41467-023-38372-1. Nat Commun. 2023. PMID: 37160921 Free PMC article. No abstract available.
Abstract
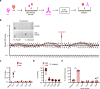
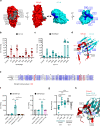
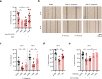
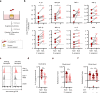
Life-threatening bacterial infections in women after childbirth, known as puerperal sepsis, resulted in classical epidemics and remain a global health problem. While outbreaks of puerperal sepsis have been ascribed to Streptococcus pyogenes, little is known about disease mechanisms. Here, we show that the bacterial R28 protein, which is epidemiologically associated with outbreaks of puerperal sepsis, specifically targets the human receptor CEACAM1. This interaction triggers events that would favor the development of puerperal sepsis, including adhesion to cervical cells, suppression of epithelial wound repair and subversion of innate immune responses. High-resolution structural analysis showed that an R28 domain with IgI3-like fold binds to the N-terminal domain of CEACAM1. Together, these findings demonstrate that a single adhesin-receptor interaction can drive the pathogenesis of bacterial sepsis and provide molecular insights into the pathogenesis of one of the most important infectious diseases in medical history.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Holmes, O. The contagiousness of puerperal fever. New Engl. Quart. J. Med.1, 503–530 (1843).
-
- Semmelweis, I. P. Die Aetiologie, der Begriff und die Prophylaxis des Kindbettfiebers, 1861. (University of Wisconsin Press, 1983).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

