Growth dynamics and amorphous-to-crystalline phase transformation in natural nacre
- PMID: 37080977
- PMCID: PMC10119311
- DOI: 10.1038/s41467-023-37814-0
Growth dynamics and amorphous-to-crystalline phase transformation in natural nacre
Abstract
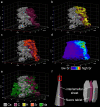
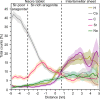
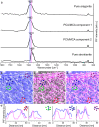
Biominerals, such as nacreous bivalve shells, are important archives of environmental information. Most marine calcifiers form their shells from amorphous calcium carbonate, hypothesised to occur via particle attachment and stepwise crystallisation of metastable precursor phases. However, the mechanism of this transformation, including the incorporation of trace elements used for environmental reconstructions, are poorly constrained. Here, using shells of the Mediterranean mussel, we explore the formation of nacre from the meso- to the atomic scale. We use a combination of strontium pulse-chase labelling experiments in aquaculture and correlated micro- to sub-nanoscale analysis to show that nacre grows in a dynamic two-step process with extensional and space-filling growth components. Furthermore, we show that nacre crystallizes via localised dissolution and reprecipitation within nanogranules. Our findings elucidate how stepwise crystallization pathways affect trace element incorporation in natural biominerals, while preserving their intricate hierarchical ultrastructure.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Addadi L, Raz S, Weiner S. Taking advantage of disorder. Amorphous calcium carbonate and its roles in biomineralization. Adv. Mater. 2003;15:959–970. doi: 10.1002/adma.200300381. - DOI
LinkOut - more resources
Full Text Sources

