Helicobacter pylori infection
- PMID: 37081005
- PMCID: PMC11558793
- DOI: 10.1038/s41572-023-00431-8
Helicobacter pylori infection
Abstract
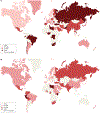
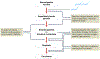
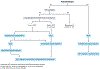
Helicobacter pylori infection causes chronic gastritis, which can progress to severe gastroduodenal pathologies, including peptic ulcer, gastric cancer and gastric mucosa-associated lymphoid tissue lymphoma. H. pylori is usually transmitted in childhood and persists for life if untreated. The infection affects around half of the population in the world but prevalence varies according to location and sanitation standards. H. pylori has unique properties to colonize gastric epithelium in an acidic environment. The pathophysiology of H. pylori infection is dependent on complex bacterial virulence mechanisms and their interaction with the host immune system and environmental factors, resulting in distinct gastritis phenotypes that determine possible progression to different gastroduodenal pathologies. The causative role of H. pylori infection in gastric cancer development presents the opportunity for preventive screen-and-treat strategies. Invasive, endoscopy-based and non-invasive methods, including breath, stool and serological tests, are used in the diagnosis of H. pylori infection. Their use depends on the specific individual patient history and local availability. H. pylori treatment consists of a strong acid suppressant in various combinations with antibiotics and/or bismuth. The dramatic increase in resistance to key antibiotics used in H. pylori eradication demands antibiotic susceptibility testing, surveillance of resistance and antibiotic stewardship.
© 2023. Springer Nature Limited.
Conflict of interest statement
Competing interests
P.M. has consulted for Aboca, Bayer Healthcare, Cinclus, Imevax, Menarini Foundation and Phatom. P.M. has received honoraria for lectures from Allergosan, Biohit, Biocodex and Malesci. S.I.S. has received scientific support from Richen. C.S. has received speaker fees from Imevax, Falk Foundation and Lilly. S.S. is listed as an inventor on a patent application related to the use of bacterial motility inhibitors as potential treatment for
Figures


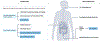
References
-
- Suerbaum S & Michetti P Helicobacter pylori infection. N. Engl. J. Med 347, 1175–1186 (2002). - PubMed
-
- Peek RM Jr & Blaser MJ Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat. Rev. Cancer 2, 28–37 (2002). - PubMed
-
- Amieva MR & El-Omar EM Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology 134, 306–323 (2008). - PubMed
-
- van Amsterdam K, van Vliet AH, Kusters JG & van der Ende A Of microbe and man: determinants of Helicobacter pylori-related diseases. FEMS Microbiol. Rev 30, 131–156 (2006). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

