Textural properties and adsorption behavior of Zn-Mg-Al layered double hydroxide upon crystal violet dye removal as a low cost, effective, and recyclable adsorbent
- PMID: 37081088
- PMCID: PMC10119303
- DOI: 10.1038/s41598-023-33142-x
Textural properties and adsorption behavior of Zn-Mg-Al layered double hydroxide upon crystal violet dye removal as a low cost, effective, and recyclable adsorbent
Abstract
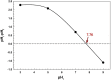
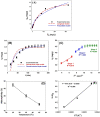
The preparation of adsorbents plays a vital role in the adsorption method. In particular, many adsorbents with high specific surface areas and unique shapes are essential for the adsorption strategy. A Zn-Mg-Al/layer double hydroxide (LDH) was designed in this study using a simple co-precipitation process. Adsorbent based on Zn-Mg-Al/LDH was used to remove crystal violet (CV) from the wastewater. The impacts of the initial dye concentration, pH, and temperature on CV adsorption performance were systematically examined. The adsorbents were analyzed both before and after adsorption using FTIR, XRD, and SEM. The roughness parameters and surface morphologies of the produced LDH were estimated using 3D SEM images. Under the best conditions (dose of adsorbent = 0.07 g and pH = 9), the maximum adsorption capacity has been achieved. Adsorption kinetics studies revealed that the reaction that led to the adsorption of CV dye onto Zn-Mg-Al/LDH was a pseudo-second-order model. Additionally, intraparticle diffusion suggests that Zn-Mg-Al/LDH has a fast diffusion constant for CV molecules (0.251 mg/(g min1/2)). Furthermore, as predicted by the Langmuir model, the maximal Zn-Mg-Al/LDH adsorption capacity of CV was 64.80 mg/g. The CV dimensionless separation factor (RL) onto Zn-Mg-Al/LDH was 0.769, indicating that adsorption was favorable. The effect of temperature was performed at 25, 35, and 45 °C in order to establish the thermodynamic parameters ∆Ho, ∆So, and ∆Go. The computed values indicated exothermic and spontaneous adsorption processes. The study presented here might be used to develop new adsorbents with enhanced adsorption capabilities for the purpose of protecting the water environment.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
Facile synthesis of carbon-coated layered double hydroxide and its comparative characterisation with Zn-Al LDH: application on crystal violet and malachite green dye adsorption-isotherm, kinetics and Box-Behnken design.Environ Sci Pollut Res Int. 2018 Oct;25(30):30236-30254. doi: 10.1007/s11356-018-3001-3. Epub 2018 Aug 28. Environ Sci Pollut Res Int. 2018. PMID: 30155633
-
Facile preparation of sisal-Fe/Zn layered double hydroxide bio-nanocomposites for the efficient removal of rifampin from aqueous solution: kinetic, equilibrium, and thermodynamic studies.Int J Phytoremediation. 2023;25(5):586-597. doi: 10.1080/15226514.2022.2093834. Epub 2022 Jul 3. Int J Phytoremediation. 2023. PMID: 35786106
-
Decontamination of crystal violet using nanocomposite adsorbent based on pine cone biochar modified with CoFe2O4/Mn-Fe LDH.Sci Rep. 2025 Apr 29;15(1):15067. doi: 10.1038/s41598-025-99549-w. Sci Rep. 2025. PMID: 40301577 Free PMC article.
-
Zn-Al layered double hydroxide supported on waste cow dung-derived biochar as a highly efficient adsorbent for anionic dye removal from contaminated water.Environ Sci Pollut Res Int. 2024 Oct;31(50):60401-60425. doi: 10.1007/s11356-024-35176-8. Epub 2024 Oct 9. Environ Sci Pollut Res Int. 2024. PMID: 39379656
-
Efficient removal of crystal violet dye from aqueous solutions using sodium hydroxide-modified avocado shells: kinetics and isotherms modeling.Water Sci Technol. 2022 Jan;85(1):433-448. doi: 10.2166/wst.2021.451. Water Sci Technol. 2022. PMID: 35050894
Cited by
-
Synergistic effect of rice husk-derived activated carbon modified by Ni/Al-layered double hydroxides for lead removal from industrial wastewater.Sci Rep. 2024 Nov 18;14(1):28411. doi: 10.1038/s41598-024-77569-2. Sci Rep. 2024. PMID: 39557906 Free PMC article.
-
Copper Immobilized on Modified LDHs as a Novel Efficient Catalytic System for Three-Component Synthesis of Pyrano[2,3-d]pyrimidine and pyrazolo[4',3':5,6]pyrano[2,3-d]pyrimidine Derivatives.ACS Omega. 2024 Feb 21;9(9):10332-10342. doi: 10.1021/acsomega.3c07913. eCollection 2024 Mar 5. ACS Omega. 2024. PMID: 38463312 Free PMC article.
-
Fabrication of a surface molecularly imprinted polymer membrane based on a single template and its application in the separation and extraction of phenytoin, phenobarbital and lamotrigine.RSC Adv. 2024 Mar 11;14(12):8353-8365. doi: 10.1039/d4ra00294f. eCollection 2024 Mar 6. RSC Adv. 2024. PMID: 38469200 Free PMC article.
-
Characterization and application of LDH with chitosan composites investigated by positron annihilation lifetime spectroscopy and surface texture for the adsorption of methyl orange.Sci Rep. 2024 Jul 17;14(1):16501. doi: 10.1038/s41598-024-65889-2. Sci Rep. 2024. PMID: 39019938 Free PMC article.
-
Carbon supported ternary layered double hydroxide nanocomposite for Fluoxetine removal and subsequent utilization of spent adsorbent as antidepressant.Sci Rep. 2024 Feb 17;14(1):3990. doi: 10.1038/s41598-024-53781-y. Sci Rep. 2024. PMID: 38368467 Free PMC article.
References
-
- Chowdhury S, Pan S, Balasubramanian R, Das P. Date palm based activated carbon for the efficient removal of organic dyes from aqueous environment. In: Naushad M, Lichtfouse E, editors. Sustainable Agriculture Reviews. Springer; 2019. pp. 247–263.
-
- Khalid A, Zubair M. A comparative study on the adsorption of Eriochrome Black T dye from aqueous solution on graphene and acid-modified graphene. Arab. J. Sci. Eng. 2018;43:2167–2179. doi: 10.1007/s13369-017-2543-x. - DOI
-
- Al-Momani F, Touraud E, Degorce-Dumas JR, Roussy J, Thomas O. Biodegradability enhancement of textile dyes and textile wastewater by VUV photolysis. J. Photochem. Photobiol. A. 2002;153:191–197. doi: 10.1016/S1010-6030(02)00298-8. - DOI
-
- Zare K, Gupta VK, Moradi O, Makhlouf ASH, Sillanpää M, Nadagouda MN, Kazemi M. A comparative study on the basis of adsorption capacity between CNTs and activated carbon as adsorbents for removal of noxious synthetic dyes: A review. J. Nanostruct. Chem. 2015;5:227–236. doi: 10.1007/s40097-015-0158-x. - DOI
LinkOut - more resources
Full Text Sources

