Human immune and gut microbial parameters associated with inter-individual variations in COVID-19 mRNA vaccine-induced immunity
- PMID: 37081096
- PMCID: PMC10119155
- DOI: 10.1038/s42003-023-04755-9
Human immune and gut microbial parameters associated with inter-individual variations in COVID-19 mRNA vaccine-induced immunity
Abstract
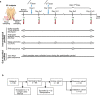
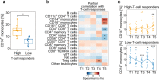
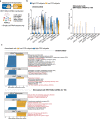
COVID-19 mRNA vaccines induce protective adaptive immunity against SARS-CoV-2 in most individuals, but there is wide variation in levels of vaccine-induced antibody and T-cell responses. However, the mechanisms underlying this inter-individual variation remain unclear. Here, using a systems biology approach based on multi-omics analyses of human blood and stool samples, we identified several factors that are associated with COVID-19 vaccine-induced adaptive immune responses. BNT162b2-induced T cell response is positively associated with late monocyte responses and inversely associated with baseline mRNA expression of activation protein 1 (AP-1) transcription factors. Interestingly, the gut microbial fucose/rhamnose degradation pathway is positively correlated with mRNA expression of AP-1, as well as a gene encoding an enzyme producing prostaglandin E2 (PGE2), which promotes AP-1 expression, and inversely correlated with BNT162b2-induced T-cell responses. These results suggest that baseline AP-1 expression, which is affected by commensal microbial activity, is a negative correlate of BNT162b2-induced T-cell responses.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

