Single-cell adaptations shape evolutionary transitions to multicellularity in green algae
- PMID: 37081145
- PMCID: PMC10250200
- DOI: 10.1038/s41559-023-02044-6
Single-cell adaptations shape evolutionary transitions to multicellularity in green algae
Abstract
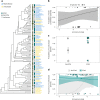
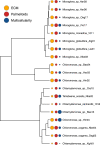
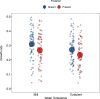
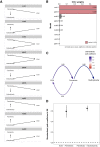
The evolution of multicellular life has played a pivotal role in shaping biological diversity. However, we know surprisingly little about the natural environmental conditions that favour the formation of multicellular groups. Here we experimentally examine how key environmental factors (predation, nitrogen and water turbulence) combine to influence multicellular group formation in 35 wild unicellular green algae strains (19 Chlorophyta species). All environmental factors induced the formation of multicellular groups (more than four cells), but there was no evidence this was adaptive, as multicellularity (% cells in groups) was not related to population growth rate under any condition. Instead, population growth was related to extracellular matrix (ECM) around single cells and palmelloid formation, a unicellular life-cycle stage where two to four cells are retained within a mother-cell wall after mitosis. ECM production increased with nitrogen levels resulting in more cells being in palmelloids and higher rates of multicellular group formation. Examining the distribution of 332 algae species across 478 lakes monitored over 55 years, showed that ECM and nitrogen availability also predicted patterns of obligate multicellularity in nature. Our results highlight that adaptations of unicellular organisms to cope with environmental challenges may be key to understanding evolutionary routes to multicellular life.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Ruiz-Trillo, I. & Nedelcu, A. M. Evolutionary Transitions to Multicellular Life: Principles and Mechanisms (Springer Berlin Heidelberg, 2015).
-
- Niklas, K. J. & Newman, S. A. Multicellularity: Origins and Evolution (MIT Press, 2016).
-
- Herron, M. D., Conlin, P. L. & Ratcliff, W. C. The Evolution of Multicellularity (Routledge, 2022).
-
- Maynard-Smith, J. & Szathmary, E. The Major Transitions in Evolution (Oxford Univ. Press, 1997).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

