Comparison of Protein Particle Formation in IgG1 mAbs Formulated with PS20 Vs. PS80 When Subjected to Interfacial Dilatational Stress
- PMID: 37081185
- PMCID: PMC10118229
- DOI: 10.1208/s12249-023-02561-4
Comparison of Protein Particle Formation in IgG1 mAbs Formulated with PS20 Vs. PS80 When Subjected to Interfacial Dilatational Stress
Abstract
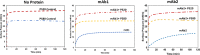
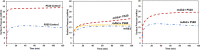
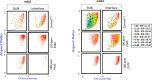
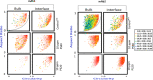
Polysorbates (PS) are nonionic surfactants that are commonly included in protein formulations to mitigate the formation of interfacial stress-induced protein particles and thus increase their long-term storage stability. Nonetheless, factors that dictate the efficiency of different polysorbates in mitigating protein particle formation, especially during the application of interfacial stresses, are often ill defined. Here, we used a Langmuir trough to determine the surface activity of two IgG1 monoclonal antibodies formulated with two different polysorbates (PS20 and PS80) when subjected to interfacial dilatational stress. Interfacial properties of these formulations were then correlated with characterization of subvisible protein particles measured by micro-flow imaging (MFI). Both mAbs, when formulated in PS20, demonstrate faster adsorption kinetics and higher surface activity compared to PS80 or surfactant-free formulations. Compression/expansion results suggest that when exposed to interfacial dilatational stresses, both mAb/PS20 formulations display interfacial properties of PS20 alone. In contrast, interfacial properties of both mAb/PS80 formulations suggest mAbs and PS80 are co-adsorbed to the air-water interface. Further, MFI analysis of the interface and the bulk solution confirms that PS20 is more effective than PS80 at mitigating the formation of larger particles in the bulk solution in both mAbs. Concomitantly, the efficiency of PS to prevent interface-induced protein particle formation also depended on the protein's inherent tendency to aggregate at a surfactant-free interface. Together, the studies presented here highlight the importance of determining the interfacial properties of mAbs, surfactants, and their combinations to make informed formulation decisions about the choice of surfactant.
Keywords: air–water interface; interface-induced protein particle formation; interfacial dilatational stress; protein aggregation.
© 2023. The Author(s), under exclusive licence to American Association of Pharmaceutical Scientists.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Understanding the Impact of Combined Hydrodynamic Shear and Interfacial Dilatational Stress, on Interface-Mediated Particle Formation for Monoclonal Antibody Formulations.J Pharm Sci. 2024 Aug;113(8):2081-2092. doi: 10.1016/j.xphs.2024.04.009. Epub 2024 Apr 12. J Pharm Sci. 2024. PMID: 38615816
-
Monoacyl phospholipids to replace polysorbates as interfacial stabilizers in parenteral monoclonal antibody formulations.Eur J Pharm Sci. 2025 Sep 1;212:107191. doi: 10.1016/j.ejps.2025.107191. Epub 2025 Jul 3. Eur J Pharm Sci. 2025. PMID: 40617264
-
Competitive Adsorption of Monoclonal Antibodies and Nonionic Surfactants at the Air-Water Interface.ACS Appl Mater Interfaces. 2025 Jul 16;17(28):40116-40128. doi: 10.1021/acsami.5c07162. Epub 2025 Jul 1. ACS Appl Mater Interfaces. 2025. PMID: 40590319
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
Cited by
-
Reduced IgG1 Antibody Left-Twisted Antiparallel β-Sheet Structure Stability Occurs under Metal-Catalyzed Oxidation Conditions in the Presence of Polysorbates.Mol Pharm. 2025 May 5;22(5):2623-2638. doi: 10.1021/acs.molpharmaceut.5c00034. Epub 2025 Apr 9. Mol Pharm. 2025. PMID: 40202920 Free PMC article.
-
Investigation on the Combined Effect of Hydroxypropyl Beta-Cyclodextrin (HPβCD) and Polysorbate in Monoclonal Antibody Formulation.Pharmaceuticals (Basel). 2024 Apr 19;17(4):528. doi: 10.3390/ph17040528. Pharmaceuticals (Basel). 2024. PMID: 38675488 Free PMC article.
References
-
- Golan BL, Quentin JB, David E. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discovery. 2020;7(1):21–39. - PubMed
-
- Lowe D et al., Aggregation, stability, and formulation of human antibody therapeutics, in Advances in Protein Chemistry and Structural Biology, R. Donev, Editor. 2011, Academic Press. p. 41–61. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

