Inhibitory control of sharp-wave ripple duration during learning in hippocampal recurrent networks
- PMID: 37081295
- PMCID: PMC10209669
- DOI: 10.1038/s41593-023-01306-7
Inhibitory control of sharp-wave ripple duration during learning in hippocampal recurrent networks
Abstract
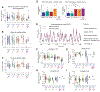
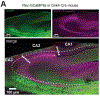
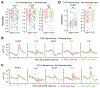
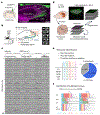
Recurrent excitatory connections in hippocampal regions CA3 and CA2 are thought to play a key role in the generation of sharp-wave ripples (SWRs), electrophysiological oscillations tightly linked with learning and memory consolidation. However, it remains unknown how defined populations of inhibitory interneurons regulate these events during behavior. Here, we use large-scale, three-dimensional calcium imaging and retrospective molecular identification in the mouse hippocampus to characterize molecularly identified CA3 and CA2 interneuron activity during SWR-associated memory consolidation and spatial navigation. We describe subtype- and region-specific responses during behaviorally distinct brain states and find that SWRs are preceded by decreased cholecystokinin-expressing interneuron activity and followed by increased parvalbumin-expressing basket cell activity. The magnitude of these dynamics correlates with both SWR duration and behavior during hippocampal-dependent learning. Together these results assign subtype- and region-specific roles for inhibitory circuits in coordinating operations and learning-related plasticity in hippocampal recurrent circuits.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

