The relaxin receptor RXFP1 signals through a mechanism of autoinhibition
- PMID: 37081311
- PMCID: PMC10530065
- DOI: 10.1038/s41589-023-01321-6
The relaxin receptor RXFP1 signals through a mechanism of autoinhibition
Abstract
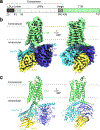
The relaxin family peptide receptor 1 (RXFP1) is the receptor for relaxin-2, an important regulator of reproductive and cardiovascular physiology. RXFP1 is a multi-domain G protein-coupled receptor (GPCR) with an ectodomain consisting of a low-density lipoprotein receptor class A (LDLa) module and leucine-rich repeats. The mechanism of RXFP1 signal transduction is clearly distinct from that of other GPCRs, but remains very poorly understood. In the present study, we determine the cryo-electron microscopy structure of active-state human RXFP1, bound to a single-chain version of the endogenous agonist relaxin-2 and the heterotrimeric Gs protein. Evolutionary coupling analysis and structure-guided functional experiments reveal that RXFP1 signals through a mechanism of autoinhibition. Our results explain how an unusual GPCR family functions, providing a path to rational drug development targeting the relaxin receptors.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests statement
A.C.K. and S.C.E. are inventors on a patent application for engineered single-chain relaxin proteins. A.C.K. is a co-founder and consultant for Tectonic Therapeutic and Seismic Therapeutic and for the Institute for Protein Innovation, a non-profit research institute. D.S.M. is a co-founder of Seismic Therapeutic, a consultant for Tectonic Therapeutic, Dyno Therapeutics, Jura Bio, Octant Bio, and a venture partner at Catalio Capital Management. The remaining authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

