Effects of multiple-dose intranasal oxytocin administration on social responsiveness in children with autism: a randomized, placebo-controlled trial
- PMID: 37081454
- PMCID: PMC10117268
- DOI: 10.1186/s13229-023-00546-5
Effects of multiple-dose intranasal oxytocin administration on social responsiveness in children with autism: a randomized, placebo-controlled trial
Abstract
Background: Intranasal administration of oxytocin is increasingly explored as a new approach to facilitate social development and reduce disability associated with a diagnosis of autism spectrum disorder (ASD). The efficacy of multiple-dose oxytocin administration in children with ASD is, however, not well established.
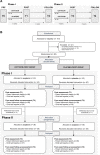
Methods: A double-blind, randomized, placebo-controlled trial with parallel design explored the effects of a 4-week intranasal oxytocin administration (12 IU, twice daily) on parent-rated social responsiveness (Social Responsiveness Scale: SRS-2) in pre-pubertal school-aged children (aged 8-12 years, 61 boys, 16 girls). Secondary outcomes included a questionnaire-based assessment of repetitive behaviors, anxiety, and attachment. Effects of oxytocin were assessed immediately after the administration period and at a follow-up, 4 weeks after the last administration. The double-blind phase was followed by a 4-week single-blind phase during which all participants received intranasal oxytocin.
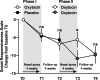
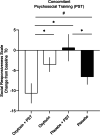
Results: In the double-blind phase, both the oxytocin and placebo group displayed significant pre-to-post-improvements in social responsiveness and secondary questionnaires, but improvements were not specific to the intranasal oxytocin. Notably, in the single-blind phase, participants who were first allocated to intranasal placebo and later changed to intranasal oxytocin displayed a significant improvement in social responsiveness, over and above the placebo-induced improvements noted in the first phase. Participants receiving oxytocin in the first phase also showed a significant further improvement upon receiving a second course of oxytocin, but only at the 4-week follow-up. Further, exploratory moderator analyses indicated that children who received psychosocial trainings (3 or more sessions per month) along with oxytocin administration displayed a more pronounced improvement in social responsiveness.
Limitations: Future studies using larger cohorts and more explicitly controlled concurrent psychosocial trainings are warranted to further explore the preliminary moderator effects, also including understudied populations within the autism spectrum, such as children with co-occurring intellectual disabilities.
Conclusions: Four weeks of oxytocin administration did not induce treatment-specific improvements in social responsiveness in school-aged children with ASD. Future studies are warranted to further explore the clinical efficacy of oxytocin administration paired with targeted psychosocial trainings that stimulate socio-communicative behaviors. Trial registration The trial was registered with the European Clinical Trial Registry (EudraCT 2018-000769-35) on June 7th, 2018 ( https://www.clinicaltrialsregister.eu/ctr-search/trial/2018-000769-35/BE ).
Keywords: Autism spectrum disorder (ASD); Oxytocin; Randomized controlled trial; Social responsiveness.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Behavioral effects of multiple-dose oxytocin treatment in autism: a randomized, placebo-controlled trial with long-term follow-up.Mol Autism. 2020 Jan 15;11(1):6. doi: 10.1186/s13229-020-0313-1. eCollection 2020. Mol Autism. 2020. PMID: 31969977 Free PMC article. Clinical Trial.
-
The effect of oxytocin nasal spray on social interaction in young children with autism: a randomized clinical trial.Mol Psychiatry. 2023 Feb;28(2):834-842. doi: 10.1038/s41380-022-01845-8. Epub 2022 Oct 27. Mol Psychiatry. 2023. PMID: 36302965 Free PMC article. Clinical Trial.
-
The effects of a course of intranasal oxytocin on social behaviors in youth diagnosed with autism spectrum disorders: a randomized controlled trial.J Child Psychol Psychiatry. 2015 Apr;56(4):444-52. doi: 10.1111/jcpp.12305. Epub 2014 Aug 2. J Child Psychol Psychiatry. 2015. PMID: 25087908 Clinical Trial.
-
Intranasal oxytocin in the treatment of autism spectrum disorders: A multilevel meta-analysis.Neurosci Biobehav Rev. 2021 Mar;122:18-27. doi: 10.1016/j.neubiorev.2020.12.028. Epub 2021 Jan 2. Neurosci Biobehav Rev. 2021. PMID: 33400920
-
Oxytocin treatment for core symptoms in children with autism spectrum disorder: a systematic review and meta-analysis.Eur J Clin Pharmacol. 2023 Oct;79(10):1357-1363. doi: 10.1007/s00228-023-03545-w. Epub 2023 Aug 4. Eur J Clin Pharmacol. 2023. PMID: 37540265
Cited by
-
Distribution of Intranasally Administered rIL-10 Along the Olfactory Nerve and Perivascular Space After Intracerebral Hemorrhage.CNS Neurosci Ther. 2025 Apr;31(4):e70372. doi: 10.1111/cns.70372. CNS Neurosci Ther. 2025. PMID: 40237247 Free PMC article.
-
Intranasal Oxytocin in Pediatric Populations: Exploring the Potential for Reducing Irritability and Modulating Neural Responses: A Mini Review.J Psychiatr Brain Sci. 2023;8(4):e230008. doi: 10.20900/jpbs.20230008. Epub 2023 Aug 31. J Psychiatr Brain Sci. 2023. PMID: 37990750 Free PMC article.
-
Oxytocin in neurodevelopmental disorders: Autism spectrum disorder and Prader-Willi syndrome.Pharmacol Ther. 2024 Dec;264:108734. doi: 10.1016/j.pharmthera.2024.108734. Epub 2024 Oct 23. Pharmacol Ther. 2024. PMID: 39455012 Free PMC article. Review.
-
Chronic oxytocin administration stimulates the oxytocinergic system in children with autism.Nat Commun. 2024 Jan 2;15(1):58. doi: 10.1038/s41467-023-44334-4. Nat Commun. 2024. PMID: 38167302 Free PMC article. Clinical Trial.
-
Cannabidiol (CBD) Treatment for Severe Problem Behaviors in Autistic Boys: A Randomized Clinical Trial.J Autism Dev Disord. 2025 May 24. doi: 10.1007/s10803-025-06884-y. Online ahead of print. J Autism Dev Disord. 2025. PMID: 40410546
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®). 5th ed. American Psychiatric Association; 2013.
-
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends Cogn Sci. 2011;15:301–309. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

