Prenatal PM2.5 exposure impairs spatial learning and memory in male mice offspring: from transcriptional regulation to neuronal morphogenesis
- PMID: 37081511
- PMCID: PMC10116824
- DOI: 10.1186/s12989-023-00520-2
Prenatal PM2.5 exposure impairs spatial learning and memory in male mice offspring: from transcriptional regulation to neuronal morphogenesis
Abstract
Background: As one of the environmental risk factors for human health, atmospheric fine particulate matter (PM2.5) contributes to cognitive deterioration in addition to respiratory and cardiovascular injuries. Recently, increasing evidence implicates that PM2.5 inhalation can affect neurological functions in offspring, but the sex-specific outcomes and the underlying biological processes are largely unknown.
Objectives: To observe the influence of prenatal PM2.5 exposure on cognitive performance in offspring, to elucidate the neuronal morphological alterations and possible transcriptional regulation based on mRNA-sequencing (mRNA-Seq) data after birth, and to determine the key components of PM2.5 contributing to the adverse effects.
Methods: Pregnant C57BL/6J mice were exposed to sterile saline or PM2.5 suspension. Morris water maze test was used to assess the cognitive function in weanling offspring. Microscopic observation was applied to detect neuronal morphogenesis in vivo and in vitro. The cortex tissues from male offspring were collected on postnatal days (PNDs) 1, 7, and 21 for mRNA-Seq analysis. The organic and inorganic components of PM2.5 were separated to assess their contributions using primary cultured neurons.
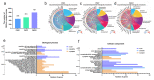
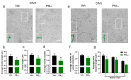
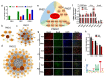
Results: Prenatal PM2.5 exposure impaired spatial learning and memory in weanling male mice, but not female mice. The sex-specific outcomes were associated with mRNA expression profiles of the cortex during postnatal critical windows, and the annotations in Gene Ontology (GO) of differentially expressed genes (DEGs) revealed that the exposure persistently disrupted the expression of genes involved in neuronal features in male offspring. Consistently, axonal growth impairment and dendritic complexity reduction were observed. Importantly, Homeobox A5 (Hoxa5), a critical transcription factor regulating all of the neuronal morphogenesis-associated hub genes on PNDs 1, 7, and 21, significantly decreased in the cortex of male offspring following PM2.5 exposure. In addition, both inorganic and organic components were harmful to axonal and dendritic growth, with organic components exhibiting stronger inhibition than inorganic ones.
Conclusion: Prenatal PM2.5 exposure affected spatial learning and memory in male mice by disrupting Hoxa5-mediated neuronal morphogenesis, and the organic components, including polycyclic aromatic hydrocarbons (PAHs), posed more adverse effects than the inorganic components.
Keywords: Fine particulate matter (PM2.5) prenatal exposure; Homeobox A5 (Hoxa5); Neuronal morphogenesis; Spatial learning and memory; mRNA expression profiles.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests.
Figures



Similar articles
-
Prenatal NO2 exposure and neurodevelopmental disorders in offspring mice: Transcriptomics reveals sex-dependent changes in cerebral gene expression.Environ Int. 2020 May;138:105659. doi: 10.1016/j.envint.2020.105659. Epub 2020 Mar 20. Environ Int. 2020. PMID: 32203807
-
Prenatal exposure to concentrated ambient PM2.5 results in spatial memory defects regulated by DNA methylation in male mice offspring.Environ Sci Pollut Res Int. 2023 Mar;30(12):35142-35152. doi: 10.1007/s11356-022-24663-5. Epub 2022 Dec 17. Environ Sci Pollut Res Int. 2023. PMID: 36526934 Free PMC article.
-
Transcriptome sequencing analysis reveals a potential role of lncRNA NONMMUT058932.2 and NONMMUT029203.2 in abnormal myelin development of male offspring following prenatal PM2.5 exposure.Sci Total Environ. 2023 Oct 15;895:165004. doi: 10.1016/j.scitotenv.2023.165004. Epub 2023 Jun 20. Sci Total Environ. 2023. PMID: 37348736
-
Adverse neurodevelopment in children associated with prenatal exposure to fine particulate matter (PM2.5) - Possible roles of polycyclic aromatic hydrocarbons (PAHs) and mechanisms involved.Reprod Toxicol. 2024 Dec;130:108718. doi: 10.1016/j.reprotox.2024.108718. Epub 2024 Sep 12. Reprod Toxicol. 2024. PMID: 39276806 Review.
-
Association between prenatal exposure to ambient particulate matter and risk of hypospadias in offspring: A systematic review and meta-analysis.Environ Res. 2021 Jan;192:110190. doi: 10.1016/j.envres.2020.110190. Epub 2020 Sep 11. Environ Res. 2021. PMID: 32919959
Cited by
-
Construction and evaluation of a diagnostic model for Alzheimer's disease based on mitophagy-related genes.Sci Rep. 2025 Mar 27;15(1):10632. doi: 10.1038/s41598-025-89980-4. Sci Rep. 2025. PMID: 40148430 Free PMC article.
-
PM2.5 Exposure Induces Glomerular Hyperfiltration in Mice in a Gender-Dependent Manner.Toxics. 2024 Dec 1;12(12):878. doi: 10.3390/toxics12120878. Toxics. 2024. PMID: 39771093 Free PMC article.
-
Developmental Toxicity of Fine Particulate Matter: Multifaceted Exploration from Epidemiological and Laboratory Perspectives.Toxics. 2024 Apr 6;12(4):274. doi: 10.3390/toxics12040274. Toxics. 2024. PMID: 38668497 Free PMC article. Review.
-
An experimental framework for conjoint measures of olfaction, navigation, and motion as pre-clinical biomarkers of Alzheimer's disease.J Alzheimers Dis Rep. 2024 Dec 23;8(1):1722-1744. doi: 10.1177/25424823241307617. eCollection 2024. J Alzheimers Dis Rep. 2024. PMID: 40034341 Free PMC article. Review.
References
-
- World Health Organization. Ambient air pollution: health impacts. 2020. http://www.who.int/airpollution/ambient/health-impacts/en/. Accessed 25 Jun 2022.
-
- Dong F, Yu B, Pan Y. Examining the synergistic effect of CO2 emissions on PM2.5 emissions reduction: evidence from china. J Clean Prod. 2019;223:759–71. doi: 10.1016/j.jclepro.2019.03.152. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

