Higher performance for women than men in MRI-based Alzheimer's disease detection
- PMID: 37081528
- PMCID: PMC10116672
- DOI: 10.1186/s13195-023-01225-6
Higher performance for women than men in MRI-based Alzheimer's disease detection
Abstract
Introduction: Although machine learning classifiers have been frequently used to detect Alzheimer's disease (AD) based on structural brain MRI data, potential bias with respect to sex and age has not yet been addressed. Here, we examine a state-of-the-art AD classifier for potential sex and age bias even in the case of balanced training data.
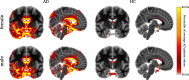
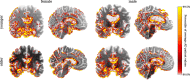
Methods: Based on an age- and sex-balanced cohort of 432 subjects (306 healthy controls, 126 subjects with AD) extracted from the ADNI data base, we trained a convolutional neural network to detect AD in MRI brain scans and performed ten different random training-validation-test splits to increase robustness of the results. Classifier decisions for single subjects were explained using layer-wise relevance propagation.
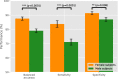
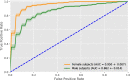
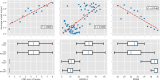
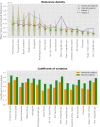
Results: The classifier performed significantly better for women (balanced accuracy [Formula: see text]) than for men ([Formula: see text]). No significant differences were found in clinical AD scores, ruling out a disparity in disease severity as a cause for the performance difference. Analysis of the explanations revealed a larger variance in regional brain areas for male subjects compared to female subjects.
Discussion: The identified sex differences cannot be attributed to an imbalanced training dataset and therefore point to the importance of examining and reporting classifier performance across population subgroups to increase transparency and algorithmic fairness. Collecting more data especially among underrepresented subgroups and balancing the dataset are important but do not always guarantee a fair outcome.
Trial registration: ClinicalTrials.gov NCT00106899.
Keywords: Alzheimer’s disease; Bias; Deep learning; MRI; Sex.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Payan A, Montana G. Predicting Alzheimer’s disease: a neuroimaging study with 3D convolutional neural networks. arXiv preprint. 2015. ArXiv:1502.02506.
-
- Wen J, Thibeau-Sutre E, Diaz-Melo M, Samper-González J, Routier A, Bottani S, et al. Convolutional neural networks for classification of Alzheimer’s disease: Overview and reproducible evaluation. Med Image Anal. 2020;63:101694. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

