Access to a novel first-line single-tablet HIV antiretroviral regimen in Affordable Care Act Marketplace plans, 2018-2020
- PMID: 37081570
- PMCID: PMC10116786
- DOI: 10.1186/s40545-023-00559-8
Access to a novel first-line single-tablet HIV antiretroviral regimen in Affordable Care Act Marketplace plans, 2018-2020
Abstract
Background: A pillar of the United States' Ending the HIV Epidemic (EHE) initiative is to rapidly provide antiretroviral therapy (ART) in order to achieve HIV viral suppression. However, insurance benefit design can impede ART access. The primary objective of this study is to understand how Affordable Care Act (ACA) Marketplace qualified health plan (QHP) formularies responded to two new ART single tablet regimens (STRs): dolutegravir/abacavir/lamivudine (DTG/ABC/3TC; approved in 2014) and bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF; approved in 2018).
Methods: We conducted a descriptive study of individual and small group QHPs to assess coverage, cost sharing (coinsurance vs. copay), specialty tiering, prior authorization, and out-of-pocket (OOP) costs for DTG/ABC/3TC and BIC/FTC/TAF. All individual and small group QHPs offered in state ACA Marketplaces from 2018-2020 were identified using plan-level formulary data from Ideon linked to end-of-year data from Robert Wood Johnson Foundation's Individual Market Health Insurance Exchange (HIX).
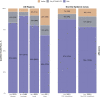
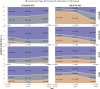
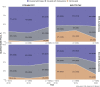
Results: For 2018, 2019, and 2020, respectively, we identified 19,533, 17,007, and 21,547 QHPs. While DTG/ABC/3TC coverage was above 91% from 2018-2020, BIC/FTC/TAF coverage improved from 60 to 86%. Coverage of BIC/FTC/TAF improved in EHE priority jurisdictions from 73 to 90% driven by increased coverage with coinsurance. Although BIC/FTC/TAF had a higher wholesale acquisition cost than DTG/ABC/3TC, monthly OOP cost trends differed regionally in the Midwest but did not differ by EHE priority jurisdiction status.
Conclusions: QHP coverage of STRs is heterogeneous across the US. While coverage of BIC/FTC/TAF increased over time, many QHPs in EHE priority jurisdictions required coinsurance. Access to new ART regimens may be slowed by delayed QHP coverage and benefit design.
Keywords: Access to care; Coinsurance; Copay; Cost; Coverage; Drug pricing; HIV; Pharmacoequity.
© 2023. The Author(s).
Conflict of interest statement
Dr. Khazanchi reports receiving grant funding from the Infectious Diseases Society of America and Boston Children’s Hospital, serving as a paid consultant to the New York City Department of Health & Mental Hygiene’s Office of the Chief Medical Officer, and serving on the strategic advisory board of the Rise to Health Coalition. Ms. Killelea reported receiving grants from Gilead Sciences, Inc. Mr. Horn and Mr. Hamp reported that NASTAD receives grant funding from Gilead Sciences Inc. and ViiV Healthcare for organizational support; all funding to NASTAD was separate from this project. Dr. McManus reports previously owning stock in Gilead Sciences, Inc; and receiving grants from the National Institute of Allergy and Infectious Diseases (NIAID). No other relevant disclosures were reported.
Figures

Similar articles
-
Comparing Real-World Healthcare Costs Associated with Single-Tablet Regimens for HIV-1: The 2-Drug Regimen Dolutegravir/Lamivudine vs. Standard 3- or 4-Drug Regimens.Infect Dis Ther. 2023 Aug;12(8):2117-2133. doi: 10.1007/s40121-023-00848-4. Epub 2023 Aug 8. Infect Dis Ther. 2023. PMID: 37552426 Free PMC article.
-
Geographic Variation in Qualified Health Plan Coverage and Prior Authorization Requirements for HIV Preexposure Prophylaxis.JAMA Netw Open. 2023 Nov 1;6(11):e2342781. doi: 10.1001/jamanetworkopen.2023.42781. JAMA Netw Open. 2023. PMID: 37948076 Free PMC article.
-
An indirect comparison of 144-week efficacy, safety, and tolerability of dolutegravir plus lamivudine and second-generation integrase inhibitor-based, 3-drug, single-tablet regimens in therapy-naive people with HIV-1.AIDS Res Ther. 2023 Mar 22;20(1):17. doi: 10.1186/s12981-023-00507-1. AIDS Res Ther. 2023. PMID: 36949442 Free PMC article.
-
Characteristics and real-world medication persistence of people living with HIV treated with DTG/3TC or BIC/FTC/TAF: a hospital claims database study in Japan.Front Med (Lausanne). 2024 Sep 10;11:1329922. doi: 10.3389/fmed.2024.1329922. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39318599 Free PMC article.
-
Comparison of the design and methodology of Phase 3 clinical trials of bictegravir/emtricitabine/tenofovir alafenamide (BIC/FTC/TAF) and dolutegravir-based dual therapy (DTG) in HIV: a systematic review of the literature.Expert Rev Anti Infect Ther. 2023 Jan;21(1):65-76. doi: 10.1080/14787210.2023.2149490. Epub 2022 Nov 27. Expert Rev Anti Infect Ther. 2023. PMID: 36399521
Cited by
-
Altered Host microRNAomics in HIV Infections: Therapeutic Potentials and Limitations.Int J Mol Sci. 2024 Aug 13;25(16):8809. doi: 10.3390/ijms25168809. Int J Mol Sci. 2024. PMID: 39201495 Free PMC article. Review.
-
Patient Attitudes Toward Self- or Partner-, Friend-, or Family-Administered Long-acting Injectable Antiretroviral Therapy: A Mixed-Methods Study Across 3 Urban Human Immunodeficiency Virus Clinics.Open Forum Infect Dis. 2024 May 13;11(6):ofae265. doi: 10.1093/ofid/ofae265. eCollection 2024 Jun. Open Forum Infect Dis. 2024. PMID: 38854389 Free PMC article.
References
-
- Kaiser family foundation. HIV Viral suppression rate in U.S. lowest among comparable high-income countries. Kaiser family foundation. 2021. https://www.kff.org/hivaids/slide/hiv-viral-suppression-rate-in-u-s-lowe.... Accessed 4 Jan 2021.
-
- Office of Infectious Disease and HIV/AIDS Policy, U.S. Department of Health & Human Services. Priority Jurisdictions: Phase I. HIV.gov. 2020. https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/jurisdictio.... Accessed 14 Feb 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
