Anti-schistosomal immunity to core xylose/fucose in N-glycans
- PMID: 37081851
- PMCID: PMC10110957
- DOI: 10.3389/fmolb.2023.1142620
Anti-schistosomal immunity to core xylose/fucose in N-glycans
Abstract
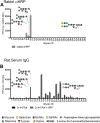
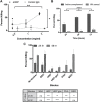
Schistosomiasis is a globally prevalent, debilitating disease that is poorly controlled by chemotherapy and for which no vaccine exists. While partial resistance in people may develop over time with repeated infections and treatments, some animals, including the brown rat (Rattus norvegicus), are only semi-permissive and have natural protection. To understand the basis of this protection, we explored the nature of the immune response in the brown rat to infection by Schistosoma mansoni. Infection leads to production of IgG to parasite glycoproteins with complex-type N-glycans that contain a non-mammalian-type modification by core α2-Xylose and core α3-Fucose (core Xyl/Fuc). These epitopes are expressed on the surfaces of schistosomula and adult worms. Importantly, IgG to these epitopes can kill schistosomula by a complement-dependent process in vitro. Additionally, sera from both infected rhesus monkey and infected brown rat were capable of killing schistosomula in a manner inhibited by glycopeptides containing core Xyl/Fuc. These results demonstrate that protective antibodies to schistosome infections in brown rats and rhesus monkeys include IgG responses to the core Xyl/Fuc epitopes in surface-expressed N-glycans, and raise the potential of novel glyco-based vaccines that might be developed to combat this disease.
Keywords: N-glycans; antigens; core fucose; core xylose; immunity; schistosomiasis.
Copyright © 2023 Prasanphanich, Leon, Secor, Shoemaker, Heimburg-Molinaro and Cummings.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources

