Shaping of the alveolar landscape by respiratory infections and long-term consequences for lung immunity
- PMID: 37081878
- PMCID: PMC10112541
- DOI: 10.3389/fimmu.2023.1149015
Shaping of the alveolar landscape by respiratory infections and long-term consequences for lung immunity
Abstract
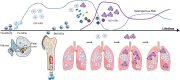
Respiratory infections and especially viral infections, along with other extrinsic environmental factors, have been shown to profoundly affect macrophage populations in the lung. In particular, alveolar macrophages (AMs) are important sentinels during respiratory infections and their disappearance opens a niche for recruited monocytes (MOs) to differentiate into resident macrophages. Although this topic is still the focus of intense debate, the phenotype and function of AMs that recolonize the niche after an inflammatory insult, such as an infection, appear to be dictated in part by their origin, but also by local and/or systemic changes that may be imprinted at the epigenetic level. Phenotypic alterations following respiratory infections have the potential to shape lung immunity for the long-term, leading to beneficial responses such as protection against allergic airway inflammation or against other infections, but also to detrimental responses when associated with the development of immunopathologies. This review reports the persistence of virus-induced functional alterations in lung macrophages, and discusses the importance of this imprinting in explaining inter-individual and lifetime immune variation.
Keywords: AM ontogeny; alveolar macrophages; lung immunity; niche imprinting; respiratory viruses; trained immunity.
Copyright © 2023 Rodriguez-Rodriguez, Gillet and Machiels.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

