The regulation of self-tolerance and the role of inflammasome molecules
- PMID: 37081890
- PMCID: PMC10110889
- DOI: 10.3389/fimmu.2023.1154552
The regulation of self-tolerance and the role of inflammasome molecules
Abstract
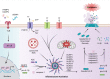
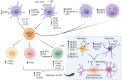
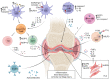
Inflammasome molecules make up a family of receptors that typically function to initiate a proinflammatory response upon infection by microbial pathogens. Dysregulation of inflammasome activity has been linked to unwanted chronic inflammation, which has also been implicated in certain autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, type 1 diabetes, systemic lupus erythematosus, and related animal models. Classical inflammasome activation-dependent events have intrinsic and extrinsic effects on both innate and adaptive immune effectors, as well as resident cells in the target tissue, which all can contribute to an autoimmune response. Recently, inflammasome molecules have also been found to regulate the differentiation and function of immune effector cells independent of classical inflammasome-activated inflammation. These alternative functions for inflammasome molecules shape the nature of the adaptive immune response, that in turn can either promote or suppress the progression of autoimmunity. In this review we will summarize the roles of inflammasome molecules in regulating self-tolerance and the development of autoimmunity.
Keywords: autoimmunity; immunoregulation; inflammasomes; inflammation; self-tolerance.
Copyright © 2023 Ke, Greenawalt, Manukonda, Ji and Tisch.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

