LZM008, a proposed tocilizumab biosimilar: Pharmacokinetics, safety, and immunogenicity profiles compared with ACTEMRA® in Chinese healthy male subjects
- PMID: 37081963
- PMCID: PMC10110837
- DOI: 10.3389/fphar.2023.1111893
LZM008, a proposed tocilizumab biosimilar: Pharmacokinetics, safety, and immunogenicity profiles compared with ACTEMRA® in Chinese healthy male subjects
Abstract
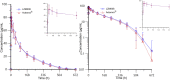
Background: This study aimed to investigate the pharmacokinetics, safety, and immunogenicity of recombinant humanized anti-human IL-6R monoclonal antibody injection, LZM008, and evaluate the pharmacokinetic similarity between LZM008 and tocilizumab (ACTEMRA®) in Chinese healthy male subjects. Research design and methods: In this randomized, double-blinded, paralleled, two-center Phase I clinical trial, 96 subjects were randomized with a 1:1 ratio to receive 4 mg/kg intravenous dose of LZM008 or ACTEMRA® and evaluated for 28 days. The pharmacokinetic bioequivalence was assessed by the maximum serum concentration (Cmax), the area under the serum concentration-time curve (AUC) from time 0 to the last detectable drug concentration (AUC0-t), and AUC0-∞. The statistical analysis was conducted using SAS Enterprise Guide statistical software. Safety was assessed by physical examinations, vital signs, laboratory tests, and electrocardiograms. Anti-drug antibodies (ADAs) were measured by a bridged electrochemiluminescence immunoassay. Results: LZM008 (N = 49) and ACTEMRA® (N = 47) groups showed similar pharmacokinetic properties. After a single intravenous infusion of 4 mg/kg LZM008, the Cmax and AUC0-∞ values of LZM008 reached 87.99 μg/mL and 11,526.70 h*μg/mL, respectively, with Tmax 1.98 h, and the half-life (t1/2) was 83.45 h. The 90% confidence intervals of ratios for Cmax, AUC0-t, and AUC0-∞ were within the range of 80.00%-125.00%. After infusion, one (2.0%) subject in the LZM008 group and three (6.4%) subjects in the ACTEMRA® group showed positive ADA test results. The incidence of treatment emergent adverse events (TEAEs) was comparable in LZM008 and ACTEMRA® groups (98.0% versus 100%), with the decrease in blood fibrinogen and neutrophil counts being the most common TEAEs. Conclusion: The pharmacokinetic characteristics and immunogenicity exhibited by LZM008 were similar to those of the reference product, ACTEMRA®. The safety profiles of LZM008 were similar in the two groups with mild-moderate adverse effects. Trial Registration: The trial is registered at www.chinadrugtrials.org.cn (CTR20190889).
Keywords: biosimilar; immunogenicity; pharmacokinetics; safety; tocilizumab.
Copyright © 2023 Cao, Wang, He, Hu, Yang, Que, Gu, Yu, Wu, Wu, Fang, He and Zhang.
Conflict of interest statement
Author WF was employed by Livzon Mabpharm Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Actemra. (2016) [Internet]. RA, GCA, SSc-ILD, SJIA, PJIA, COVID-19 Treatment | ACTEMRA® (tocilizumab) ACTEMRA; Genentech USA. http://www. actemra.com.[Accessed Nov 20, 2022].
-
- Administration F. A. D. (2015) Scientific considerations in demonstrating biosimilarity to a reference product. Food and Drug Administration; Rockville, MD: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformati... [Accessed Nov 20, 2022].
-
- Agency E. M. (2014) Guideline on similar biological medicinal products.Clin. Mol. Allergy 13 http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guidelin.... (Assessed Nov 20, 2022).
-
- Bergman G. J., Hochberg M. C., Boers M., Wintfeld N., Kielhorn A., Jansen J. P. (2010). Indirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs. Semin. Arthritis Rheum. 39, 425–441. 10.1016/j.semarthrit.2009.12.002 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials