Jinhong decoction protects sepsis-associated acute lung injury by reducing intestinal bacterial translocation and improving gut microbial homeostasis
- PMID: 37081964
- PMCID: PMC10110981
- DOI: 10.3389/fphar.2023.1079482
Jinhong decoction protects sepsis-associated acute lung injury by reducing intestinal bacterial translocation and improving gut microbial homeostasis
Abstract
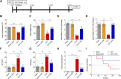
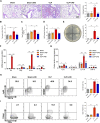
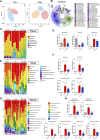
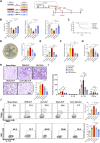
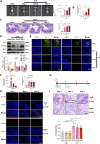
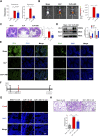
Background: Currently no specific treatments are available for sepsis and the associated syndromes including acute lung injury (ALI). Jinhong Decoction (JHD) is a traditional Chinese prescription, and it has been applied clinically as an efficient and safe treatment for sepsis, but the underlying mechanism remains unknown. The aim of the study was to explore the potential mechanisms of JHD ameliorating sepsis and concurrent ALI. Methods: The cecum ligation puncture (CLP)- induced murine sepsis model was established for determining the efficacy of JHD protecting CLP and ALI. The role of gut microbiota involved in the efficacy of JHD was evaluated by 16S rRNA sequencing and fecal microbiota transplantation (FMT). Translocation of intestinal Escherichia coli (E. coli) to lungs after CLP was verified by qPCR and in vivo-imaging. Intestinal permeability was analyzed by detecting FITC-dextran leakness. Junction proteins were evaluated by Western blotting and immunofluorescence. Results: JHD treatment remarkably increased survival rate of septic mice and alleviated sepsis-associated lung inflammation and injury. FMT suggested that the protective role for JHD was mediated through the regulation of gut microbiota. We further revealed that JHD administration partially restored the diversity and configuration of microbiome that was distorted by CLP operation. Of interest, the intestinal bacteria, E. coli particularly, was found to translocate into the lungs upon CLP via disrupting the intestinal mucosal barrier, leading to the inflammatory response and tissue damage in lungs. JHD impeded the migration and hence lung accumulation of intestinal E. coli, and thereby prevented severe ALI associated with sepsis. This effect is causatively related with the ability of JHD to restore intestinal barrier by up-regulating tight junctions. Conclusion: Our study unveils a mechanism whereby the migration of gut bacteria leads to sepsis-associated ALI, and we demonstrate the potential of JHD as an effective strategy to block this bacterial migration for treating sepsis and the associated immunopathology in the distal organs.
Keywords: JHD; acute lung injury; bacterial translocation; gut microbiome; sepsis.
Copyright © 2023 Bao, Wang, Liu, Zhang, Jin, Zhang and Shi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Adipose-derived mesenchymal stem cells attenuate acute lung injury and improve the gut microbiota in septic rats.Stem Cell Res Ther. 2020 Sep 7;11(1):384. doi: 10.1186/s13287-020-01902-5. Stem Cell Res Ther. 2020. PMID: 32894198 Free PMC article.
-
Fecal Microbiota Transplantation Protects the Intestinal Mucosal Barrier by Reconstructing the Gut Microbiota in a Murine Model of Sepsis.Front Cell Infect Microbiol. 2021 Sep 22;11:736204. doi: 10.3389/fcimb.2021.736204. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34631604 Free PMC article.
-
Jinhong decoction ameliorates injury in septic mice without disrupting the equilibrium of gut microbiota.J Pharm Biomed Anal. 2024 Dec 15;251:116404. doi: 10.1016/j.jpba.2024.116404. Epub 2024 Aug 9. J Pharm Biomed Anal. 2024. PMID: 39154578
-
Gut-Lung Crosstalk in Sepsis-Induced Acute Lung Injury.Front Microbiol. 2021 Dec 23;12:779620. doi: 10.3389/fmicb.2021.779620. eCollection 2021. Front Microbiol. 2021. PMID: 35003009 Free PMC article. Review.
-
Intestinal Mucosal Immune Barrier: A Powerful Firewall Against Severe Acute Pancreatitis-Associated Acute Lung Injury via the Gut-Lung Axis.J Inflamm Res. 2024 Apr 10;17:2173-2193. doi: 10.2147/JIR.S448819. eCollection 2024. J Inflamm Res. 2024. PMID: 38617383 Free PMC article. Review.
Cited by
-
Gut Escherichia coli promotes lung cancer by increasing circulating STAMBP production.Discov Oncol. 2025 Apr 3;16(1):459. doi: 10.1007/s12672-025-02206-x. Discov Oncol. 2025. PMID: 40180620 Free PMC article.
-
Therapeutic role of gut microbiota in lung injury-related cognitive impairment.Front Nutr. 2025 Feb 13;11:1521214. doi: 10.3389/fnut.2024.1521214. eCollection 2024. Front Nutr. 2025. PMID: 40017811 Free PMC article. Review.
-
Role of gut microbes in acute lung injury/acute respiratory distress syndrome.Gut Microbes. 2024 Jan-Dec;16(1):2440125. doi: 10.1080/19490976.2024.2440125. Epub 2024 Dec 10. Gut Microbes. 2024. PMID: 39658851 Free PMC article. Review.
-
Exploring the Anti-PANoptosis Mechanism of Dachaihu Decoction Against Sepsis-Induced Acute Lung Injury: Network Pharmacology, Bioinformatics, and Experimental Validation.Drug Des Devel Ther. 2025 Jan 17;19:349-368. doi: 10.2147/DDDT.S495225. eCollection 2025. Drug Des Devel Ther. 2025. PMID: 39839500 Free PMC article.
-
Crosstalk between lung and extrapulmonary organs in sepsis-related acute lung injury/acute respiratory distress syndrome.Ann Intensive Care. 2025 Jul 14;15(1):97. doi: 10.1186/s13613-025-01513-4. Ann Intensive Care. 2025. PMID: 40658295 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

