The antimicrobial peptide Polybia-MP1 differentiates membranes with the hopanoid, diplopterol from those with cholesterol
- PMID: 37082019
- PMCID: PMC10074923
- DOI: 10.1016/j.bbadva.2021.100002
The antimicrobial peptide Polybia-MP1 differentiates membranes with the hopanoid, diplopterol from those with cholesterol
Abstract
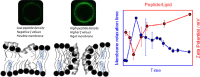
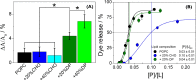
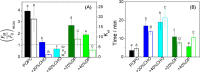
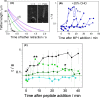
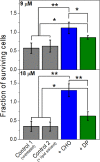
Polybia-MP1 is an antimicrobial peptide that shows a decreased activity in membranes with cholesterol (CHO). Since it is now accepted that hopanoids act as sterol-surrogates in some sterol-lacking bacteria, we here inquire about the impact of Polybia-MP1 on membranes containing the hopanoid diplopterol (DP) in comparison to membranes with CHO. We found that, despite the properties induced on lipid membranes by DP are similar to those induced by CHO, the effect of Polybia-MP1 on membranes with CHO or DP was significantly different. DP did not prevent dye release from LUVs, nor the insertion of Polybia-MP1 into monolayers, and peptide-membrane affinity was higher for those with DP than with CHO. Zeta potentials ( ) for DP-containing LUVs showed a complex behavior at increasing peptide concentration. The effect of the peptide on membrane elasticity, investigated by nanotube retraction experiments, showed that peptide addition softened all membrane compositions, but membranes with DP got stiffer at long times. Considering this, and the results, we propose that peptides accumulate at the interface adopting different arrangements, leading to a non-monotonic behavior. Possible correlations with cell membranes were inquired testing the antimicrobial activity of Polybia-MP1 against hopanoid-lacking bacteria pre-incubated with DP or CHO. The fraction of surviving cells was lower in cultures incubated with DP compared to those incubated with CHO. We propose that the higher activity of Polybia-MP1 against some bacteria compared to mammalian cells is not only related to membrane electrostatics, but also the composition of neutral lipids, particularly the hopanoids, could be important.
Keywords: Bacterial resistance; Lytic activity; Membrane dynamic elasticity; Optical tweezers; Targeting of AMPs; Zeta potential.
© 2021 The Authors.
Figures



Similar articles
-
Phosphatidylserine lipids and membrane order precisely regulate the activity of Polybia-MP1 peptide.Biochim Biophys Acta Biomembr. 2017 Jun;1859(6):1067-1074. doi: 10.1016/j.bbamem.2017.03.002. Epub 2017 Mar 6. Biochim Biophys Acta Biomembr. 2017. PMID: 28274844
-
The insertion of Polybia-MP1 peptide into phospholipid monolayers is regulated by its anionic nature and phase state.Chem Phys Lipids. 2017 Oct;207(Pt A):38-48. doi: 10.1016/j.chemphyslip.2017.08.001. Epub 2017 Aug 10. Chem Phys Lipids. 2017. PMID: 28802697
-
The effect of acidic pH on the adsorption and lytic activity of the peptides Polybia-MP1 and its histidine-containing analog in anionic lipid membrane: a biophysical study by molecular dynamics and spectroscopy.Amino Acids. 2021 May;53(5):753-767. doi: 10.1007/s00726-021-02982-0. Epub 2021 Apr 22. Amino Acids. 2021. PMID: 33890127
-
Chemical approaches in the development of natural nontoxic peptide Polybia-MP1 as a potential dual antimicrobial and antitumor agent.Amino Acids. 2021 Jun;53(6):843-852. doi: 10.1007/s00726-021-02995-9. Epub 2021 May 4. Amino Acids. 2021. PMID: 33948731 Review.
-
Hopanoid lipids: from membranes to plant-bacteria interactions.Nat Rev Microbiol. 2018 May;16(5):304-315. doi: 10.1038/nrmicro.2017.173. Epub 2018 Feb 19. Nat Rev Microbiol. 2018. PMID: 29456243 Free PMC article. Review.
Cited by
-
Wasp Venom: Future Breakthrough in Production of Antimicrobial Peptides.Protein J. 2025 Feb;44(1):35-47. doi: 10.1007/s10930-024-10242-9. Epub 2024 Dec 4. Protein J. 2025. PMID: 39633224 Review.
-
Multiple roles of ribosomal antimicrobial peptides in tackling global antimicrobial resistance.R Soc Open Sci. 2022 Jan 26;9(1):211583. doi: 10.1098/rsos.211583. eCollection 2022 Jan. R Soc Open Sci. 2022. PMID: 35116161 Free PMC article.
-
Investigating the effects of pcDNA3 polybia-MP1 on the apoptosis induction in lung cancer cell line.Sci Rep. 2025 Aug 2;15(1):28226. doi: 10.1038/s41598-025-13439-9. Sci Rep. 2025. PMID: 40753292 Free PMC article.
-
Selectivity of membrane-active peptides: the role of electrostatics and other membrane biophysical properties.Biophys Rev. 2025 Apr 10;17(2):591-604. doi: 10.1007/s12551-025-01309-7. eCollection 2025 Apr. Biophys Rev. 2025. PMID: 40376421 Review.
-
On the Coupling between Mechanical Properties and Electrostatics in Biological Membranes.Membranes (Basel). 2021 Jun 28;11(7):478. doi: 10.3390/membranes11070478. Membranes (Basel). 2021. PMID: 34203412 Free PMC article. Review.
References
-
- Heimburg T. WILEY-VCH Verlag GmbH - Co.KGaA; 2007. Thermal Biophysics of Membranes.
-
- Bloom M., Mourttsen O.G. The evolution of membranes. Handb. Biol. Phys. 1995;1(C):65–95. doi: 10.1016/S1383-8121(06)80019-9. - DOI
-
- Demel R., de Kruyff B. The function of sterols in membranes. Biochim. Biophys. Acta. 1976;13:258–283. - PubMed
LinkOut - more resources
Full Text Sources

