Treatment outcomes of mucosal melanoma of head and neck: Efficacy of immune checkpoint inhibitors for advanced disease
- PMID: 37082097
- PMCID: PMC10112385
- DOI: 10.3389/fsurg.2022.1032626
Treatment outcomes of mucosal melanoma of head and neck: Efficacy of immune checkpoint inhibitors for advanced disease
Abstract
Background: Head and neck mucosal melanoma (HNMM) is a rare and aggressive subtype of melanoma. HNMM often develops as a recurrent or metastatic disease, and its prognosis is worse than that of cutaneous melanoma. Recent large-scale clinical studies have reported favorable outcomes with immune checkpoint inhibitors (ICIs) for melanoma. However, these clinical trials included only a small number of HNMM cases. This study aimed to estimate treatment outcomes and prognostic predictors of ICIs for advanced HNMM.
Methods: Cases of advanced HNMM, defined as unresectable or metastatic HNMM at the initial diagnosis (five patients) or development of recurrent/metastatic HNMM after initial treatment (27 patients), were included in this study. Survival analysis and a search for prognostic factors were performed for these 32 patients. Furthermore, the detailed clinical course of patients who received ICI treatment was investigated.
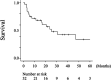
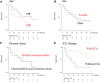
Results: The median overall survival (OS) of 32 patients with advanced HNMM was 25.3 months. The estimated 1-, 3-, and 5-year OS rates were 68.4%, 42.8%, and 34.3%, respectively. Fourteen patients (43.7%) received ICIs, whereas 18 (56.3%) did not. Univariate analysis showed that ICI treatment was the only factor associated with a better 1-year OS. Patients who received ICI treatment had significantly longer OS (median OS: not reached, 1-year OS: 85.7%) than those who did not (median OS: 11.3 months, 1-year OS: 54.5%). The overall response and disease control rates of patients who received ICI treatment were 50% and 64.3%, respectively. Patients who achieved complete response (CR) or partial response (PR) to ICI treatment survived significantly longer (1-year OS: 100%) than those who did not (1-year OS: 71.4%). Among the five patients who discontinued ICI treatment due to severe immune-related adverse events (irAEs), four did not receive salvage treatments but showed durable treatment effects and survived for 9.8-54.2 months at the end of the follow-up period.
Conclusions: ICI treatment achieved a favorable OS for advanced HNMM. CR/PR to ICI treatment and discontinuation owing to severe irAEs were favorable predictors of OS.
Keywords: Advanced disease; Head and neck mucosal melanoma; Immune checkpoint inhibitor; Immune-related adverse events; Overall Response Rate (ORR).
© 2022 Ohshima, Ueki, Yokoyama, Takahashi, Shodo, Yamazaki, Okabe, Matsuyama, Togashi, Takatsuka, Takenouchi and Horii.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Many Chang AE, Karnell LH, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. (1998) 83:1664–78. 10.1002/(sici)1097-0142(19981015)83:8<1664::aid-cncr23>3.0.co;2-g - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

