Impact of the treatment crossover design on comparative efficacy in EMPOWER-Lung 1: Cemiplimab monotherapy as first-line treatment of advanced non-small cell lung cancer
- PMID: 37082098
- PMCID: PMC10110970
- DOI: 10.3389/fonc.2022.1081729
Impact of the treatment crossover design on comparative efficacy in EMPOWER-Lung 1: Cemiplimab monotherapy as first-line treatment of advanced non-small cell lung cancer
Abstract
Objectives: In randomized-controlled crossover design trials, overall survival (OS) treatment effect estimates are often confounded by the control group benefiting from treatment received post-progression. We estimated the adjusted OS treatment effect in EMPOWER-Lung 1 (NCT03088540) by accounting for the potential impact of crossover to cemiplimab among controls and continued cemiplimab treatment post-progression.
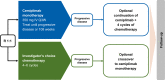
Methods: Patients were randomly assigned 1:1 to cemiplimab 350 mg every 3 weeks (Q3W) or platinum-doublet chemotherapy. Patients with disease progression while on or after chemotherapy could receive cemiplimab 350 mg Q3W for ≤108 weeks. Those who experienced progression on cemiplimab could continue cemiplimab at 350 mg Q3W for ≤108 additional weeks with four chemotherapy cycles added. Three adjustment methods accounted for crossover and/or continued treatment: simplified two-stage correction (with or without recensoring), inverse probability of censoring weighting (IPCW), and rank-preserving structural failure time model (RPSFT; with or without recensoring).
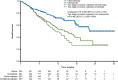
Results: In the programmed cell death-ligand 1 ≥50% population (N=563; median 10.8-month follow-up), 38.2% (n=107/280) crossed over from chemotherapy to cemiplimab (71.3%, n=107/150, among those with confirmed progression) and 16.3% (n=46/283) received cemiplimab treatment after progression with the addition of histology-specific chemotherapy (38.7%, n=46/119, among those with confirmed progression). The unadjusted OS hazard ratio (HR) with cemiplimab versus chemotherapy was 0.566 (95% confidence interval [CI]: 0.418, 0.767). Simplified two-stage correction-the most suitable method based on published guidelines and trial characteristics-produced an OS HR of 0.490 (95% CI: 0.365, 0.654) without recensoring and 0.493 (95% CI: 0.361, 0.674) with recensoring. The IPCW and RPSFT methods produced estimates generally consistent with simplified two-stage correction.
Conclusions: After adjusting for treatment crossover and continued cemiplimab treatment after progression with the addition of histology-specific chemotherapy observed in EMPOWER-Lung 1, cemiplimab continued to demonstrate a clinically important and statistically significant OS benefit versus chemotherapy, consistent with the primary analysis.
Keywords: EMPOWER-lung 1; cemiplimab; chemotherapy; crossover design; first-line treatment; non-small cell lung cancer.
Copyright © 2023 Feliciano, McLoone, Xu, Quek, Kuznik, Pouliot, Gullo, Rietschel, Guyot, Konidaris, Chan, Keeping, Wilson and Freemantle.
Conflict of interest statement
NF declares consulting services for Abbott Singapore, ALK, Allergan, Aimmune, AstraZeneca, Galderma, Ipsen, Novartis, Novo Nordisk, Regeneron, Sanofi Aventis, Thea, and Vertex; and a leadership or fiduciary role for the European Association of Cardiothoracic Surgery. JF has received grants or contracts from Bristol-Myers Squibb, AstraZeneca and Pfizer; consulting fees from AstraZeneca, Coherus, Eli Lilly, Genentech, Merck, Pfizer, Regeneron, and Takeda; honoraria from Janssen; and meeting attendance support from Regeneron. YX, RQ, AK, J-FP, GG, and PR are employees of Regeneron Pharmaceuticals. PG and GK are employees of Sanofi. DL, KC, SK, and FW are employees of PRECISIONheor and received funding from Regeneron Pharmaceuticals and Sanofi to produce this work.
Figures
Similar articles
-
First-line cemiplimab monotherapy and continued cemiplimab beyond progression plus chemotherapy for advanced non-small-cell lung cancer with PD-L1 50% or more (EMPOWER-Lung 1): 35-month follow-up from a mutlicentre, open-label, randomised, phase 3 trial.Lancet Oncol. 2023 Sep;24(9):989-1001. doi: 10.1016/S1470-2045(23)00329-7. Epub 2023 Aug 14. Lancet Oncol. 2023. PMID: 37591293 Clinical Trial.
-
Cemiplimab Plus Chemotherapy Versus Chemotherapy Alone in Advanced NSCLC: 2-Year Follow-Up From the Phase 3 EMPOWER-Lung 3 Part 2 Trial.J Thorac Oncol. 2023 Jun;18(6):755-768. doi: 10.1016/j.jtho.2023.03.008. Epub 2023 Mar 29. J Thorac Oncol. 2023. PMID: 37001859 Clinical Trial.
-
Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial.Lancet. 2021 Feb 13;397(10274):592-604. doi: 10.1016/S0140-6736(21)00228-2. Lancet. 2021. PMID: 33581821 Clinical Trial.
-
Safety, efficacy, and quality of life with cemiplimab treatment among non-small cell lung cancer patients: a systematic review and meta-analysis.Ann Med Surg (Lond). 2025 Apr 25;87(6):3800-3809. doi: 10.1097/MS9.0000000000003329. eCollection 2025 Jun. Ann Med Surg (Lond). 2025. PMID: 40486556 Free PMC article. Review.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
Cited by
-
MRI-based radiomics feature combined with tumor markers to predict TN staging of rectal cancer.J Robot Surg. 2024 May 29;18(1):229. doi: 10.1007/s11701-024-01978-8. J Robot Surg. 2024. PMID: 38809383
-
Nomograms constructed for predicting diagnosis and prognosis in cervical cancer patients with second primary malignancies: a SEER database analysis.J Cancer Res Clin Oncol. 2023 Nov;149(14):13201-13210. doi: 10.1007/s00432-023-05192-1. Epub 2023 Jul 21. J Cancer Res Clin Oncol. 2023. PMID: 37479758 Free PMC article.
-
Dual-energy CT in musculoskeletal imaging: technical considerations and clinical applications.Radiol Med. 2024 Jul;129(7):1038-1047. doi: 10.1007/s11547-024-01827-6. Epub 2024 May 14. Radiol Med. 2024. PMID: 38743319 Free PMC article. Review.
-
Risk prediction of second primary malignancies in primary colorectal neuroendocrine neoplasms patients: a population-based study.J Endocrinol Invest. 2023 Sep;46(9):1881-1889. doi: 10.1007/s40618-023-02047-x. Epub 2023 Mar 4. J Endocrinol Invest. 2023. PMID: 36870016
-
Phenotype-dependent heterogeneity of THSD7A expression in gastric cancer tissue in a patient with THSD7A-associated membranous nephropathy.CEN Case Rep. 2025 Jun 9. doi: 10.1007/s13730-025-01006-0. Online ahead of print. CEN Case Rep. 2025. PMID: 40489000
References
-
- Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. . Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (2021) 397(10274):592–604. doi: 10.1016/s0140-6736(21)00228-2 - DOI - PubMed
-
- Regeneron Pharmaceuticals, Inc . FDA approves Libtayo® (cemiplimab-rwlc) monotherapy for patients with first-line advanced non-small cell lung cancer with PD-L1 expression of ≥50% (2021). Available at: https://investor.regeneron.com/news-releases/news-release-details/fda-ap....
-
- Regeneron Pharmaceuticals, Inc . LIBTAYO® (cemiplimab-rwlc) injection, for intravenous use [US prescribing information] (2021). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761097s007lbl.pdf.
-
- Freemantle N, Xu Y, Wilson FR, Guyot P, Chen CI, Keeping S, et al. . Network meta-analysis of immune-oncology monotherapy as first-line treatment for advanced non-small-cell lung cancer in patients with PD-L1 expression ≥50. Ther Adv Med Oncol (2022) 14:17588359221105024. doi: 10.1177/17588359221105024 - DOI - PMC - PubMed
-
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. . Updated analysis of KEYNOTE-024: Pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. J Clin Oncol (2019) 37(7):537–46. doi: 10.1200/JCO.18.00149 - DOI - PubMed
LinkOut - more resources
Full Text Sources

