Combination CD200R/PD-1 blockade in a humanised mouse model
- PMID: 37082107
- PMCID: PMC10112683
- DOI: 10.1093/immadv/ltad006
Combination CD200R/PD-1 blockade in a humanised mouse model
Abstract
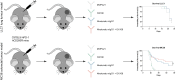
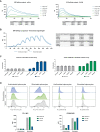
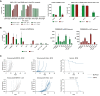
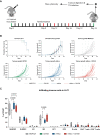
There is an increasing number of immune-checkpoint inhibitors being developed and approved for cancer immunotherapy. Most of the new therapies aim to reactivate tumour-infiltrating T cells, which are responsible for tumour killing. However, in many tumours, the most abundant infiltrating immune cells are macrophages and myeloid cells, which can be tumour-promoting as well as tumouricidal. CD200R was initially identified as a myeloid-restricted, inhibitory immune receptor, but was subsequently also found to be expressed within the lymphoid lineage. Using a mouse model humanised for CD200R and PD-1, we investigated the potential of a combination therapy comprising nivolumab, a clinically approved PD-1 blocking antibody, and OX108, a CD200R antagonist. We produced nivolumab as a murine IgG1 antibody and validated its binding activity in vitro as well as ex vivo. We then tested the combination therapy in the immunogenic colorectal cancer model MC38 as well as the PD-1 blockade-resistant lung cancer model LLC1, which is characterised by a large number of infiltrating myeloid cells, making it an attractive target for CD200R blockade. No significant improvement of overall survival was found in either model, compared to nivolumab mIgG1 monotherapy. There was a trend for more complete responses in the MC38 model, but investigation of the infiltrating immune cells failed to account for this. Importantly, MC38 cells expressed low levels of CD200, whereas LLC1 cells were CD200-negative. Further investigation of CD200R-blocking antibodies in tumours expressing high levels of CD200 could be warranted.
Keywords: CD200R; PD-1; cancer immunotherapy; monoclonal antibody; nivolumab.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
Similar articles
-
CD200R blockade enhances anti-tumor immunity by unleashing NK and CD8+ T cells in tumor.Acta Pharmacol Sin. 2025 May 6. doi: 10.1038/s41401-025-01556-0. Online ahead of print. Acta Pharmacol Sin. 2025. PMID: 40329005
-
CD200 Blockade Modulates Tumor Immune Microenvironment but Fails to Show Efficacy in Inhibiting Tumor Growth in a Murine Model of Melanoma.Front Cell Dev Biol. 2021 Oct 8;9:739816. doi: 10.3389/fcell.2021.739816. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34692697 Free PMC article.
-
Different mechanisms of CD200-CD200R induce diverse outcomes in cancer treatment.Math Biosci. 2023 Nov;365:109072. doi: 10.1016/j.mbs.2023.109072. Epub 2023 Sep 19. Math Biosci. 2023. PMID: 37734537
-
Cancel cancer: The immunotherapeutic potential of CD200/CD200R blockade.Front Oncol. 2023 Jan 23;13:1088038. doi: 10.3389/fonc.2023.1088038. eCollection 2023. Front Oncol. 2023. PMID: 36756156 Free PMC article. Review.
-
CD200-CD200R Pathway in the Regulation of Tumor Immune Microenvironment and Immunotherapy.Adv Exp Med Biol. 2020;1223:155-165. doi: 10.1007/978-3-030-35582-1_8. Adv Exp Med Biol. 2020. PMID: 32030689 Free PMC article. Review.
Cited by
-
Translation of Data from Animal Models of Cancer to Immunotherapy of Breast Cancer and Chronic Lymphocytic Leukemia.Genes (Basel). 2024 Feb 25;15(3):292. doi: 10.3390/genes15030292. Genes (Basel). 2024. PMID: 38540350 Free PMC article.
-
CD200/CD200R: Bidirectional Role in Cancer Progression and Immunotherapy.Biomedicines. 2023 Dec 16;11(12):3326. doi: 10.3390/biomedicines11123326. Biomedicines. 2023. PMID: 38137547 Free PMC article. Review.
-
Comprehensive immunophenotyping reveals distinct tumor microenvironment alterations in anti-PD-1 sensitive and resistant syngeneic mouse model.Sci Rep. 2025 Mar 10;15(1):8311. doi: 10.1038/s41598-025-91979-w. Sci Rep. 2025. PMID: 40064915 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
