Enantioselective Metal-Catalyzed Reductive Coupling of Alkynes with Carbonyl Compounds and Imines: Convergent Construction of Allylic Alcohols and Amines
- PMID: 37082110
- PMCID: PMC10112658
- DOI: 10.1021/acscatal.2c02444
Enantioselective Metal-Catalyzed Reductive Coupling of Alkynes with Carbonyl Compounds and Imines: Convergent Construction of Allylic Alcohols and Amines
Abstract
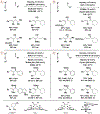
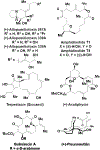
The use of alkynes as vinylmetal pronucleophiles in intermolecular enantioselective metal-catalyzed carbonyl and imine reductive couplings to form allylic alcohols and amines is surveyed. Related hydrogen auto-transfer processes, wherein alcohols or amines serve dually as reductants and carbonyl or imine proelectrophiles, also are cataloged, as are applications in target-oriented synthesis. These processes represent an emerging alternative to the use of stoichiometric vinylmetal reagents or Nozaki-Hiyama-Kishi (NHK) reactions in carbonyl and imine alkenylation.
Keywords: Alkyne; Allylic Alcohol; Allylic Amine; Enantioselective; Hydrogen Transfer; Reductive Coupling.
Figures
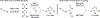


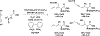

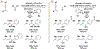

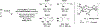
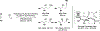

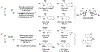
References
-
- For an introduction to the broad topic of carbonyl and imine addition, see: Comprehensive Organic Synthesis, 2nd ed.; Knochel P; Molander GA, Eds.; Elsevier: Oxford, 2014; Vols. 1 and 2.
-
- For selected reviews on the catalytic enantioselective addition of vinyl anions to carbonyl compounds and imines, see: Wipf P; Kendall C Novel Applications of Alkenyl Zirconocenes. Chem. Eur. J 2002, 8, 1778–1784. - PubMed
- Kauffman MC; Walsh PJ In Science of Synthesis: Stereoselective Synthesis; Molander GA, Ed.; Georg Thieme Verlag KG: Stuttgart, 2011; Vol. 2, pp 449–495.
- Lumbroso A; Cooke ML; Breit B Catalytic Asymmetric Synthesis of Allylic Alcohols and Derivatives and their Applications in Organic Synthesis. Angew. Chem. Int. Ed 2013, 52, 1890–1932. - PubMed
-
- For a recent review on catalytic enantioselective vinylzinc additions to aldehydes, see: Bauer T Enantioselective Dialkylzinc-Mediated Alkynylation, Arylation and Alkenylation of Carbonyl Groups. Coord. Chem. Rev 2015, 299, 83–150.
-
- For selected examples of catalytic enantioselective vinylzinc additions to aldehydes, see: Oppolzer W; Radinov RN Catalytic Asymmetric Synthesis of Secondary (E)-Allyl Alcohols from Acetylenes and Aldehydes via (1-Alkenyl)zinc Intermediates. Helv. Chim. Acta 1992, 75, 170–173.
- Oppolzer W; Radinov RN Synthesis of (R)-(−)-Muscone by an Asymmetrically Catalyzed Macrocyclization of an ω-Alkynal. J. Am. Chem. Soc 1993, 115, 1593–1594.
- Soai K; Takahashi K Asymmetric Alkenylation of Chiral and Prochiral Aldehydes Catalysed by Chiral or Achiral Amino Alcohols: Catalytic Diastereoselective Synthesis of Protected erythro-Sphingosine and Enantioselective Synthesis of Chiral Diallyl Alcohols. J. Chem. Soc., Perkin Trans 1 1994, 1257–1258.
- Oppolzer W; Radinov RN; De Brabander J Total Synthesis of the Macrolide (+)-Aspicilin by an Asymmetrically Catalyzed Macrocyclization of an ω-Alkynal Ester. Tetrahedron Lett 1995, 36, 2607–2610.
- Wipf P; Ribe S Zirconocene-Zinc Transmetalation and in Situ Catalytic Asymmetric Addition to Aldehydes. J. Org. Chem 1998, 63, 6454–6455.
- Oppolzer W; Radinov RN; El-Sayed E Catalytic Asymmetric Synthesis of Macrocyclic (E)-Allylic Alcohols from ω-Alkynals via Intramolecular 1-Alkenylzinc/Aldehyde Additions. J. Org. Chem 2001, 66, 4766–4770. - PubMed
- Dahmen S; Bräse S [2,2]Paracyclophane-Based N,O-Ligands in Alkenylzinc Additions to Aldehydes. Org. Lett 2001, 3, 4119–4122. - PubMed
- Chen YK; Lurain AE; Walsh PJ A General, Highly Enantioselective Method for the Synthesis of D and L α-Amino Acids and Allylic Amines. J. Am. Chem. Soc 2002, 124, 12225–12231. - PubMed
- Ji J-X; Qiu L-Q; Yip CW; Chan ASC A Convenient, One-Step Synthesis of Optically Active Tertiary Aminonaphthol and Its Applications in the Highly Enantioselective Alkenylations of Aldehydes. J. Org. Chem 2003, 68, 1589–1590. - PubMed
- Lurain AE; Walsh PJ A Catalytic Asymmetric Method for the Synthesis of γ-Unsaturated β-Amino Acid Derivatives. J. Am. Chem. Soc 2003, 125, 10677–10683. - PubMed
- Jeon S-J; Chen YK; Walsh PJ Catalytic Asymmetric Synthesis of Hydroxy Enol Ethers: Approach to a Two-Carbon Homologation of Aldehydes. Org. Lett 2005, 7, 1729–1732. - PubMed
- Lauterwasser F; Gall J; Höfener S; Bräse S Second-Generation N,O-[2.2]Paracyclophane Ketimine Ligands for the Alkenylzinc Addition to Aliphatic and Aromatic Aldehydes: Scope and Limitations. Adv. Synth. Catal 2006, 348, 2068–2074.
- Salvi L; Jeon S-J; Fisher EL; Carroll PJ; Walsh PJ Catalytic Asymmetric Generation of (Z)-Disubstituted Allylic Alcohols. J. Am. Chem. Soc 2007, 129, 16119–16125. - PMC - PubMed
- DeBerardinis AM; Turlington M; Pu L Activation of Vinyl Iodides for the Highly Enantioselective Addition to Aldehydes. Angew. Chem. Int. Ed 2011, 50, 2368–2370. - PubMed
-
- For selected reviews on enantioselective Nozaki-Hiyama-Kishi vinylations, see: Hargaden GC; Guiry PJ The Development of the Asymmetric Nozaki–Hiyama–Kishi Reaction. Adv. Synth. Catal 2007, 349, 2407–2424.
- Tian Q; Zhang G Recent Advances in the Asymmetric Nozaki–Hiyama–Kishi Reaction. Synthesis 2016, 48, 4038–4049.
- Gil A; Albericio F; AÁlvarez M Role of the Nozaki–Hiyama–Takai–Kishi Reaction in the Synthesis of Natural Products. Chem. Rev 2017, 117, 8420–8446. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
