A Common Active Intermediate in the Oxidation of Alkenes, Alcohols and Alkanes with H2O2 and a Mn(II)/Pyridin-2-Carboxylato Catalyst
- PMID: 37082112
- PMCID: PMC10108234
- DOI: 10.1002/cctc.202201072
A Common Active Intermediate in the Oxidation of Alkenes, Alcohols and Alkanes with H2O2 and a Mn(II)/Pyridin-2-Carboxylato Catalyst
Abstract
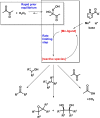
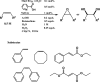
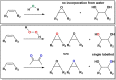
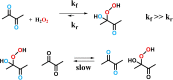
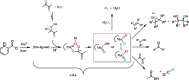
The mechanism and the reactive species involved in the oxidation of alkenes, and alcohols with H2O2, catalysed by an in situ prepared mixture of a MnII salt, pyridine-2-carboxylic acid and a ketone is elucidated using substrate competition experiments, kinetic isotope effect (KIE) measurements, and atom tracking with 18O labelling. The data indicate that a single reactive species engages in the oxidation of both alkenes and alcohols. The primary KIE in the oxidation of benzyl alcohols is ca. 3.5 and shows the reactive species to be selective despite a zero order dependence on substrate concentration, and the high turnover frequencies (up to 30 s-1) observed. Selective 18O labelling identifies the origin of the oxygen atoms transferred to the substrate during oxidation, and is consistent with a highly reactive, e. g., [MnV(O)(OH)] or [MnV(O)2], species rather than an alkylperoxy or hydroperoxy species.
Keywords: alkene; isotope labelling; kinetic isotope effect; manganese; oxidation chemistry.
© 2022 The Authors. ChemCatChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Highly efficient alkane oxidation catalyzed by [Mn(V)(N)(CN)4](2-). Evidence for [Mn(VII)(N)(O)(CN)4](2-) as an active intermediate.J Am Chem Soc. 2014 May 28;136(21):7680-7. doi: 10.1021/ja5019546. Epub 2014 May 13. J Am Chem Soc. 2014. PMID: 24799179
-
Mechanism of cis-dihydroxylation and epoxidation of alkenes by highly H(2)O(2) efficient dinuclear manganese catalysts.Inorg Chem. 2007 Aug 6;46(16):6353-72. doi: 10.1021/ic7003613. Epub 2007 Jul 3. Inorg Chem. 2007. PMID: 17608415
-
Kinetic isotope effects as probes of the mechanism of galactose oxidase.Biochemistry. 1998 Jun 9;37(23):8426-36. doi: 10.1021/bi980328t. Biochemistry. 1998. PMID: 9622494
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
-
An evaluation of structural models for the photosynthetic water-oxidizing complex derived from spectroscopic and X-ray diffraction signatures.J Biol Inorg Chem. 2002 Jan;7(1-2):2-22. doi: 10.1007/s00775-001-0305-3. Epub 2001 Nov 8. J Biol Inorg Chem. 2002. PMID: 11862536 Review.
Cited by
-
Realising the ambivalent nature of H2O2 in oxidation catalysis - its dual role as an oxidant and a substrate.Dalton Trans. 2025 Jul 1;54(26):10207-10215. doi: 10.1039/d5dt01106j. Dalton Trans. 2025. PMID: 40539534 Free PMC article. Review.
-
Dependence of O2 Depletion on Transition Metal Catalyst in Radical Polymerization of Cross-Linking Alkene Resins.Inorg Chem. 2025 Apr 21;64(15):7716-7725. doi: 10.1021/acs.inorgchem.5c00760. Epub 2025 Apr 5. Inorg Chem. 2025. PMID: 40186563 Free PMC article.
References
-
- Bäckvall J., Modern Agric. Sci. Technol., Wiley, Weinheim, Germany, Germany, 2010.
-
- Philip R. M., Radhika S., Abdulla C. M. A., Anilkumar G., Adv. Synth. Catal. 2021, 363, 1272–1289.
-
- Tseberlidis G., Demonti L., Pirovano V., Scavini M., Cappelli S., Rizzato S., Vicente R., Caselli A., ChemCatChem 2019, 11, 4907–4915.
-
- Vicens L., Costas M., Dalton Trans. 2018, 47, 1755–1763. - PubMed
-
- Bryliakov K. P., Chem. Rev. 2017, 117, 11406–11459. - PubMed
LinkOut - more resources
Full Text Sources
